Key Points
Chemotherapy results in a short median time to next treatment in patients with mycosis fungoides/Sézary syndrome.
α-interferon achieves a superior time to next treatment compared with chemotherapy, regardless of stage.
Abstract
Numerous systemic treatment options exist for patients with mycosis fungoides (MF) and Sézary syndrome (SS), but no large comparative studies are published. To study the efficacy of treatments, a retrospective analysis of our cutaneous lymphoma database was undertaken, with 198 MF/SS patients undergoing systemic therapies. The primary end point was time to next treatment (TTNT). Patients with advanced-stage disease made up 53%. The median follow-up time from diagnosis for all alive patients was 4.9 years (range 0.3-39.6), with a median survival of 11.4 years. Patients received a median of 3 lines of therapy (range 1-13), resulting in 709 treatment episodes. Twenty-eight treatment modalities were analyzed. The median TTNT for single- or multiagent chemotherapy was only 3.9 months (95% confidence interval [CI] 3.2-5.1), with few durable remissions. α-interferon gave a median TTNT of 8.7 months (95% CI 6.0-18.0), and histone deacetylase inhibitors (HDACi) gave a median TTNT of 4.5 months (95% CI 4.0-6.1). When compared directly with chemotherapy, interferon and HDACi both had greater TTNT (P < .00001 and P = .01, respectively). This study confirms that all chemotherapy regimens assessed have very modest efficacy; we recommend their use be restricted until other options are exhausted.
Introduction
Mycosis fungoides (MF) and Sézary syndrome (SS) are the most common forms of cutaneous T-cell lymphoma (CTCL), with an approximate incidence of 0.3 to 1 per 100 000 annually.1-3 Patients are classified as having either early-stage (patches/plaques) or advanced-stage (tumors, erythroderma, nodal and/or visceral involvement)4,5 disease. Moreover, additional prognostic markers are frequently used.5-8 CTCL remains incurable and therefore treatment is primarily aimed at improving symptoms and quality of life and maintaining remission.
Multiple treatment options exist for CTCL, and treatment decisions must take into account patient disease characteristics, comorbidities, treatment availability, and potential toxicities of treatment. Consequently, although there are published treatment guidelines,9-11 management approaches vary considerably for patients requiring systemic therapy. Moreover, because of the relative rarity of the disease, consensus guidelines are based on limited evidence, with no comparative studies across treatments,12 and rather are shaped by the experience of leading clinicians.13
For patients with early-stage disease, treatment generally begins with skin-directed therapies (SDT) such as psoralen plus UVA radiation (PUVA), narrowband UVB (nbUVB) radiation, topical corticosteroids, and sometimes topical chemotherapies. Local radiotherapy is also commonly used. Patients with advanced-stage disease or early-stage disease that is not controlled by SDT are usually started on systemic treatments. Many chemotherapeutic agents have demonstrable activity in MF/SS, though no single regimen has been identified as superior and remission duration is variably reported (Table 1).14-25 Some investigators prefer multiagent chemotherapy in the hope that these may have superior response rates (RRs), whereas others prefer to use single-agent therapy with the aim of reducing toxicity, particularly infection, a significant complication in patients with MF/SS.26
Major clinical studies of systemic chemotherapy in MF/SS
| Therapy . | Study type . | Efficacy . | Durability . |
|---|---|---|---|
| EPOCH | NR14 | ORR stage IIB-IV: 80% | PFS: 8 mo |
| CHOP-based | NR15 | ORR stage IIB: 66% | |
| Fludarabine plus α-interferon | NR16 | ORR stage IIA-IVA: 58% | PFS: 5.9 mo |
| ORR stage IVB: 40% | |||
| Fludarabine plus cyclophosphamide | NR17 | ORR stage IIB-III: 55% | DOR: 10 mo |
| Gemcitabine | NR18 | CR: 22% | Duration of CR: 10 mo |
| NR19 | CR: 11.5% | Duration of CR: 15 mo | |
| NR20 | ORR: 51% | DFI: 15-120 mo | |
| Pegylated liposomal doxorubicin | RA21 | ORR stage IA-IV: 88% | DFS: 13.3 mo |
| NR25 | ORR: 41% | DOR: 6 mo | |
| Low-dose methotrexate | RA22 | ORR T2 disease: 33% | TTF: 15 mo |
| RA23 | ORR T4 disease: 58% | TTF: 31 mo | |
| Pralatrexate | NR24 | ORR transformed disease: 25% | PFS: 1.7 mo |
| Therapy . | Study type . | Efficacy . | Durability . |
|---|---|---|---|
| EPOCH | NR14 | ORR stage IIB-IV: 80% | PFS: 8 mo |
| CHOP-based | NR15 | ORR stage IIB: 66% | |
| Fludarabine plus α-interferon | NR16 | ORR stage IIA-IVA: 58% | PFS: 5.9 mo |
| ORR stage IVB: 40% | |||
| Fludarabine plus cyclophosphamide | NR17 | ORR stage IIB-III: 55% | DOR: 10 mo |
| Gemcitabine | NR18 | CR: 22% | Duration of CR: 10 mo |
| NR19 | CR: 11.5% | Duration of CR: 15 mo | |
| NR20 | ORR: 51% | DFI: 15-120 mo | |
| Pegylated liposomal doxorubicin | RA21 | ORR stage IA-IV: 88% | DFS: 13.3 mo |
| NR25 | ORR: 41% | DOR: 6 mo | |
| Low-dose methotrexate | RA22 | ORR T2 disease: 33% | TTF: 15 mo |
| RA23 | ORR T4 disease: 58% | TTF: 31 mo | |
| Pralatrexate | NR24 | ORR transformed disease: 25% | PFS: 1.7 mo |
CHOP, cyclophosphamide, doxorubicin, vincristine, and prednisolone; CR, complete response; DFI, disease-free interval; DFS, disease-free survival; EPOCH, etoposide, vincristine, doxorubicin, cyclophosphamide, and prednisolone; NR, nonrandomized; RA, retrospective analysis; TTF, time-to-treatment failure.
Nonchemotherapeutic novel agents such as α-interferon,27 the rexinoid bexarotene,28,29 histone deacetylase inhibitors (HDACi) (ie, vorinostat,30,31 romidepsin,32 panobinostat,33,34 and belinostat35 ), the monoclonal antibody alemtuzumab,36,37 and the fusion toxin denileukin diftitox38-40 and extracorporeal photopheresis (ECP),41 have all shown activity in MF/SS. Moreover, high-dose chemotherapy with autologous hematopoietic stem cell transplant (AuSCT) has a high overall RR (ORR), but responses are usually short-lived.42 Conversely, allogeneic hematopoietic stem cell transplant (AlloSCT) is appealing because there is a proven graft-versus-CTCL effect.43-46 However, because the median age of MF/SS is approximately 70 years,1 this latter strategy is only applicable to a small minority of patients.
One key issue in evaluating and comparing the efficacy of various treatments is the determination of a clinically meaningful end point in CTCL, especially in the context of a retrospective analysis. Relapse appraisal relies heavily on the evaluation of the extent of skin disease and is now routinely evaluated by the modified severity-weighted assessment tool (mSWAT) in prospective studies.47 However, this measurement tool has only recently been adopted and thus is of limited use in a retrospective analysis. Progression-free survival (PFS) and duration of response (DOR) are additional frequently reported end points.5,47 However, these too rely on the quality of skin evaluation, and as such are difficult to assess and validate outside the settings of prospective clinical trials. Moreover, unlike in systemic lymphomas where progression is easily defined and usually clinically relevant, “progression” in the context of MF/SS is often described as a worsening to a more advanced stage (upstaging). Although upstaging may be a useful measure when a patient progresses in an overt way such as the development of tumors (T3; IIB), subtle progression such as that from 9% body surface area involvement (T1 disease) to 12% (T2 disease) is defined as upstaging despite its minimal clinical significance. In addition to this, large cell transformation is another mark of progression in MF/SS and infers a poor prognosis.48
Length of time to next treatment (TTNT) was selected as our primary outcome because it provides a functional and clinically relevant measure of the effectiveness of therapy. TTNT implies a durability of response that we believe is particularly meaningful in a usually indolent disease like MF/SS, where progression may be subtle and driven by symptomatic development in addition to objective measurement of tumor activity. Indeed, TTNT is an easily reproducible objective end point for retrospective analysis and has been effectively applied to the assessment of treatment efficacy in other hematologic malignancies.49-51
In this study, we set out to retrospectively evaluate the comparative efficacy of systemic therapy modalities in a large cohort of patients with MF/SS.
Methods
Study design
Retrospective analysis of our cutaneous lymphoma database at the Peter MacCallum Cancer Centre and St. Vincent’s Hospital, Melbourne, Australia, was undertaken, containing 547 patients with MF/SS presenting over a 39-year period. Data collection and analysis were approved in accordance with the Human Research Ethics Committee at our institution. Data collected included patient demographics and clinical and pathologic findings, including disease stage, tumor-node-metastasis-blood (TNMB) status and history of large-cell transformation. The database did not uniformly document treatment toxicities. During analysis, in some cases, insufficient information was available to accurately record the TNMB status (particularly blood [B] status) or dates of a specific treatment; in these cases, treatments were recorded with missing details.
Study population
Patients included for analysis had a biopsy-proven diagnosis of MF or SS requiring at least 1 systemic therapy. Of the 547 patients with MF/SS in the database, 326 were excluded for analysis because they either had not required any active treatment or had only undergone SDT. From the remaining 221 patients, 23 were excluded because of insufficient evidence for a definitive diagnosis of MF/SS or insufficient information was available. A final cohort of 198 patients with MF (n = 134) or SS (n = 64) was entered into the analysis (supplemental Figure 1, available on the Blood Web site).
Systemic therapies were defined as IV or oral chemotherapy (including AuSCT and AlloSCT), biological response modifiers, immunotherapy, PUVA, total skin electron beam radiation (TSEB), and ECP. PUVA was included as a systemic therapy in accordance with current clinical guidelines and clinical trial criteria for CTCL. In instances where multiple treatments were given simultaneously or in an overlapping manner, the primary treatment modality was selected to represent that therapeutic strategy.
Systemic corticosteroid was excluded as a new episode of therapy if its sole indication was the treatment of “disease flare” occurring in the context of initiation of a new therapy.
Thirteen chemotherapy regimens were identified including HyperCVAD (alternating cyclophosphamide/doxorubicin-based with cytarabine/methotrexate52 ), CHOP-like (cyclophosphamide, doxorubicin, vincristine, prednisolone) regimens, non-CHOP “salvage” regimens (listed in Table 2), gemcitabine-based chemotherapy, fludarabine-based chemotherapy, other purine analogs, high-dose methotrexate (dose range 0.5-3 g/m2), low-dose methotrexate (defined as oral doses up to 30 mg weekly), chlorambucil, cyclophosphamide-based treatment, single-agent anthracycline, mitozantrone-based treatment, bendamustine, and etoposide.
Chemotherapy TTNT analysis
| . | N . | Median TTNT (months) . | TTNT 95% CI (months) . | 1-y free from further treatment (%) . | 2-y free from further treatment (%) . | Median line of therapy . |
|---|---|---|---|---|---|---|
| All chemotherapy | 144 | 3.9 | 3.2-5.1 | 10.7 | 5.4 | 4 |
| Hyper CVAD | 13 | 5.0 | 2.6-5.5 | 7.7 | — | 2 |
| CHOP-like regimens | 28 | 5.7 | 2.3-7.7 | 14.3 | 14.3 | 2.5 |
| Non-CHOP “salvage” regimens* | 13 | 2.5 | 1.7-3.7 | 7.7 | — | 6 |
| Gemcitabine-based therapy | 30 | 4.0 | 3.0-5.7 | 11.1 | — | 5 |
| Fludarabine-based therapy | 8 | 2.3 | 0.3-7.4 | 12.5 | — | 5.5 |
| Chlorambucil | 14 | 4.8 | 2.1-9.0 | 21.4 | 21.4 | 2 |
| Cyclophosphamide-based therapy | 20 | 3.8 | 2.7-5.9 | 10.5 | — | 4 |
| Mitozantrone-based therapy | 3 | 5.8 | 2.5-N/A | 0 | 0 | 3 |
| Single-agent anthracycline | 6 | 1.8 | 0.7-N/A | — | — | 5 |
| Bendamustine | 1 | N/A | N/A | 0 | 0 | 4 |
| Etoposide | 3 | 3.2 | 1.6-N/A | 0 | 0 | 6 |
| Other purine analogs | 6 | 3.9 | 2.0-N/A | 0 | 0 | 7 |
| . | N . | Median TTNT (months) . | TTNT 95% CI (months) . | 1-y free from further treatment (%) . | 2-y free from further treatment (%) . | Median line of therapy . |
|---|---|---|---|---|---|---|
| All chemotherapy | 144 | 3.9 | 3.2-5.1 | 10.7 | 5.4 | 4 |
| Hyper CVAD | 13 | 5.0 | 2.6-5.5 | 7.7 | — | 2 |
| CHOP-like regimens | 28 | 5.7 | 2.3-7.7 | 14.3 | 14.3 | 2.5 |
| Non-CHOP “salvage” regimens* | 13 | 2.5 | 1.7-3.7 | 7.7 | — | 6 |
| Gemcitabine-based therapy | 30 | 4.0 | 3.0-5.7 | 11.1 | — | 5 |
| Fludarabine-based therapy | 8 | 2.3 | 0.3-7.4 | 12.5 | — | 5.5 |
| Chlorambucil | 14 | 4.8 | 2.1-9.0 | 21.4 | 21.4 | 2 |
| Cyclophosphamide-based therapy | 20 | 3.8 | 2.7-5.9 | 10.5 | — | 4 |
| Mitozantrone-based therapy | 3 | 5.8 | 2.5-N/A | 0 | 0 | 3 |
| Single-agent anthracycline | 6 | 1.8 | 0.7-N/A | — | — | 5 |
| Bendamustine | 1 | N/A | N/A | 0 | 0 | 4 |
| Etoposide | 3 | 3.2 | 1.6-N/A | 0 | 0 | 6 |
| Other purine analogs | 6 | 3.9 | 2.0-N/A | 0 | 0 | 7 |
Hyper CVAD Course A, cyclophosphamide, vincistrine, doxorubicin, dexamethasone; Hyper CVAD Course B, methotrexate, cytarabine; N/A, not able to be assessed.
Non-CHOP “salvage” regimens include: CMED (cyclophosphamide, methotrexate, etoposide, dexamethasone), DHAC (dexamethasone, doxorubicin, cytarabine, carboplatin), DHAP (dexamethasone, cytarabine, cisplatin), EPOCH (etoposide, prednisolone, vincristine, cyclophosphamide, doxorubicin), ICE (ifosfamide, carboplatin, etoposide), and Hi-DICE (dexamethasone, ifosfamide, cisplatin, etoposide).
For the purposes of comparative analysis, chemotherapy regimens were assessed separately and together in an amalgamated group. This amalgamated chemotherapy group included all chemotherapy options except for high-dose methotrexate (primarily used as a single-agent regimen to reduce tumor bulk before ECP or TSEB, rather than as a standalone regimen). Low-dose methotrexate, as well as AuSCT and AlloSCT, were each analyzed separately from other chemotherapies and were also not included in amalgamated analyses.
Other treatment modalities individually assessed were PUVA, systemic corticosteroids, α-interferon, HDACi, bexarotene, alemtuzumab, denileukin diftitox, TSEB, ECP, leflunomide, lenalidomide, and bortezomib-based treatment.
For analysis, lines of therapy were defined as lines of systemic therapy only. Treatment line was divided into first-line, mid-line (including 2nd-4th lines), and late-line (including all treatments used at 5th line or later).
Data analysis
The study close-out date was either February 17, 2014 or the last known follow-up before this date for survival, palliation, or death of the patient. Statistical analyses were performed using the Stata statistical software (version 13; StataCorp LP, College Station, TX). Survival was calculated from the date of initial CTCL diagnosis and from the date of first systemic therapy.
TTNT was calculated as the duration between the date of treatment commencement and the date of commencement of the following systemic treatment. Where patients did not have a subsequent treatment, the TTNT was censored to, as appropriate, one of the following: date of commencement of palliation, date of death, or date of last follow-up/close-out. Maintenance therapy, as defined as new therapy introduced after an induction regimen, was not generally used in this patient population, and TTNT analysis was calculated from therapy commencement dates only.
Time-to-event analyses were analyzed using the Kaplan-Meier method with intergroup comparisons using the log-rank test. Population characteristics were compared using χ2 tests for categorical variables and Wilcoxon-Mann-Whitney tests for continuous variables. A P value <.05 was considered significant.
Results
Total patient cohort
The median follow-up time from diagnosis for all alive MF/SS patients receiving systemic therapy was 4.9 years (range 0.3-39.6). During the period of study analysis, 75 patients died (37.9%), with a median survival of 11.4 years (95% confidence interval [CI] 7.4-19.0) from diagnosis and 9.5 years (95% CI 5.7-11.8) from first systemic therapy.
The clinical characteristics of all patients are summarized in Table 3. The median age at diagnosis was 61.8 years and the male:female ratio was 1.3:1. One-hundred thirty-four patients (67.7%) had a diagnosis of MF and 64 (32.3%) had a diagnosis of SS. Thirteen patients (6.5%) had folliculotropic variant disease. One-hundred five patients (53.0%) had advanced-stage (IIB-IVB) disease at diagnosis; the largest group was stage IVA (n = 58; 29.3%).
Patient characteristics of key treatment groups
| . | Total cohort . | Chemotherapy . | α-interferon . | HDACi . | Bexarotene . | Alemtuzumab . | Denileukin diftitox . | Autologous SCT . | Allogeneic SCT . | ECP . | TSEB . |
|---|---|---|---|---|---|---|---|---|---|---|---|
| . | 198 . | 144 . | 68 . | 74 . | 20 . | 16 . | 22 . | 19 . | 9 . | 53 . | 65 . |
| Gender | |||||||||||
| Male | 112 (56.6) | 71 (49.3) | 34 (50.0) | 40 (54.1) | 15 (75.0) | 7 (43.7) | 13 (59.1) | 10 (52.6) | 5 (55.6) | 29 (54.7) | 34 (52.3) |
| Female | 86 (43.4) | 73 (50.7) | 34 (50.0) | 34 (45.9) | 5 (25.0) | 9 (56.3) | 9 (40.9) | 9 (47.4) | 4 (44.4) | 24 (45.3) | 31 (47.7) |
| Diagnosis | |||||||||||
| MF | 134 (67.7) | 105 (72.9) | 47 (69.1) | 53 (71.6) | 13 (65.0) | 5 (31.2) | 16 (72.7) | 14 (73.7) | 5 (55.6) | 10 (18.9) | 48 (73.8) |
| SS | 64 (32.3) | 39 (27.1) | 21 (30.9) | 21 (28.4) | 7 (35.0) | 11 (68.8) | 6 (27.3) | 5 (26.3) | 4 (44.4) | 43 (81.1) | 17 (26.2) |
| Stage at diagnosis | |||||||||||
| Early | 93 (43.0) | 66 (45.8) | 32 (47.1) | 35 (47.3) | 11 (55.0) | 2 (12.5) | 13 (59.1) | 5 (26.3) | 2 (22.2) | 3 (5.7) | 37 (56.9) |
| Advanced | 105 (57.0) | 78 (54.2) | 36 (52.9) | 39 (52.7) | 9 (45.0) | 14 (87.5) | 9 (40.9) | 14 (73.7) | 7 (77.8) | 50 (94.3) | 28 (43.1) |
| 1A | 51 (25.8) | 31 (21.5) | 12 (17.6) | 15 (20.3) | 5 (25.0) | 0 (0) | 8 (36.4) | 2 (10.5) | 0 (0) | 1 (1.9) | 14 (21.5) |
| 1B | 41 (20.7) | 35 (24.3) | 20 (29.4) | 19 (25.7) | 6 (30.0) | 2 (12.5) | 5 (22.7) | 3 (15.8) | 2 (22.2) | 2 (3.8) | 23 (35.4) |
| 2A | 1 (0.5) | 0 (0) | 0 (0) | 1 (1.4) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) |
| 2B | 30 (15.2) | 35 (24.3) | 12 (17.7) | 13 (17.6) | 0 (0) | 3 (18.8) | 4 (18.2) | 5 (26.3) | 2 (22.2) | 0 (0) | 11 (16.9) |
| 3A | 6 (3.0) | 2 (1.4) | 3 (4.4) | 3 (4.0) | 1 (5.0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 3 (5.7) | 3 (4.6) |
| 3B | 8 (4.0) | 0 (0) | 3 (4.4) | 1 (1.4) | 1 (5.0) | 1 (6.2) | 0 (0) | 1 (5.3) | 2 (22.2) | 6 (11.3) | 0 (0) |
| 4A | 58 (29.3) | 38 (26.4) | 18 (26.5) | 21 (28.4) | 6 (30.0) | 10 (62.5) | 5 (22.7) | 6 (31.6) | 3 (33.3) | 41 (77.3) | 13 (20.0) |
| 4B | 3 (1.5) | 3 (2.1) | 0 (0) | 1 (1.4) | 1 (5.0) | 0 (0) | 0 (0) | 2 (10.5) | 0 (0) | 0 (0) | 1 (1.5) |
| Folliculotropic | |||||||||||
| Non-FT | 185 (93.5) | 141 (97.9) | 60 (88.2) | 66 (89.2) | 19 (95.0) | 16 (100) | 21 (95.5) | 17 (89.5) | 8 (88.9) | 53 (100) | 61 (93.8) |
| FT | 13 (6.5) | 3 (2.1) | 8 (11.8) | 8 (10.8) | 1 (5.0) | 0(0) | 1 (4.5) | 2 (10.5) | 1 (11.1) | 0 (0) | 4 (6.2) |
| Tumor (skin) score at treatment commencement | |||||||||||
| T1 | 33 (4.7) | 3 (2.2) | 5 (7.3) | 2 (2.7) | 1 (5.0) | 0 (0) | 1 (4.5) | 1 (5.6) | 1 (12.5) | 0 (0) | 2 (3.1) |
| T2 | 244 (35.0) | 40 (28.8) | 31 (45.6) | 24 (32.4) | 9 (45.0) | 2 (12.5) | 8 (36.4) | 4 (22.2) | 2 (25.0) | 6 (11.3) | 27 (42.2) |
| T3 | 168 (24.1) | 53 (38.1) | 11 (16.2) | 21 (28.4) | 2 (10.0) | 2 (12.5) | 5 (22.7) | 8 (44.4) | 3 (37.5) | 0 (0) | 20 (31.3) |
| T4 | 252 (36.2) | 43 (30.9) | 21 (30.9) | 27 (36.5) | 8 (40.0) | 12 (75.0) | 8 (36.4) | 5 (27.8) | 2 (25.0) | 47 (88.7) | 15 (23.4) |
| Nodal involvement at treatment commencement | |||||||||||
| N0 | 487 (69.9) | 82 (60.3) | 51 (77.3) | 52 (70.3) | 14 (70.0) | 9 (56.2) | 16 (76.2) | 7 (41.2) | 7 (87.5) | 35 (66.0) | 53 (82.8) |
| N1, N2, N3, Nx | 210 (30.1) | 54 (39.7) | 15 (22.7) | 22 (29.7) | 6 (30.0) | 7 (43.8) | 5 (23.8) | 10 (58.8) | 1 (12.5) | 18 (34.0) | 11 (17.2) |
| Visceral involvement at treatment commencement | |||||||||||
| M0 | 680 (97.4) | 133 (95.7) | 68 (100) | 73 (98.7) | 19 (95.0) | 16 (100) | 21 (95.5) | 14 (77.8) | 8 (100) | 52 (98.1) | 63 (96.9) |
| M1 | 18 (2.6) | 6 (4.3) | 0 (0) | 1 (1.3) | 1 (5.0) | 0 (0) | 1 (4.5) | 4 (22.2) | 0 (0) | 1 (1.9) | 2 (3.1) |
| Blood involvement at treatment commencement | |||||||||||
| B0 | 484 (69.4) | 102 (73.4) | 49 (72.1) | 54 (73.0) | 16 (80.0) | 7 (43.8) | 18 (81.8) | 11 (61.1) | 5 (62.5) | 10 (18.9) | 52 (80.0) |
| B1 | 38 (5.5) | 1 (0.7) | 3 (4.4) | 5 (6.7) | 0 (0) | 0 (0) | 1 (4.6) | 2 (11.1) | 1 (12.5) | 8 (15.1) | 4 (6.1) |
| B2 | 175 (25.1) | 36 (25.9) | 16 (23.5) | 15 (20.3) | 4 (20.0) | 9 (56.2) | 3 (13.6) | 5 (27.8) | 2 (25.0) | 35 (66.0) | 9 (13.9) |
| LCT at treatment commencement | |||||||||||
| Non-LCT | 615 (86.7) | 106 (73.6) | 64 (94.1) | 60 (81.1) | 18 (90.0) | 16 (100) | 20 (90.9) | 11 (57.9) | 8 (88.9) | 53 (100) | 62 (95.4) |
| LCT | 94 (13.3) | 38 (26.4) | 4 (5.9) | 14 (18.9) | 2 (10.0) | 0 (0) | 2 (9.1) | 8 (42.1) | 1 (11.1) | 0 (0) | 3 (4.6) |
| Median age at diagnosis, y | 61.8 | 56.8 | 57.8 | 58.0 | 64.6 | 57.7 | 60.7 | 58.9 | 40.6 | 64.7 | 67.4 |
| Median age at treatment, y | 62.5 | 62.6 | 60.3 | 63.4 | 66.6 | 61.5 | 62.9 | 59.9 | 42.1 | 65.8 | 67.7 |
| Median treatment line (range) | 3 (1-13) | 4 (1-12) | 2 (1-9) | 3 (1-9) | 2 (1-11) | 3.5 (1-8) | 4 (1-11) | 3 (1-11) | 6 (2-10) | 2 (1-11) | 2 (1-8) |
| . | Total cohort . | Chemotherapy . | α-interferon . | HDACi . | Bexarotene . | Alemtuzumab . | Denileukin diftitox . | Autologous SCT . | Allogeneic SCT . | ECP . | TSEB . |
|---|---|---|---|---|---|---|---|---|---|---|---|
| . | 198 . | 144 . | 68 . | 74 . | 20 . | 16 . | 22 . | 19 . | 9 . | 53 . | 65 . |
| Gender | |||||||||||
| Male | 112 (56.6) | 71 (49.3) | 34 (50.0) | 40 (54.1) | 15 (75.0) | 7 (43.7) | 13 (59.1) | 10 (52.6) | 5 (55.6) | 29 (54.7) | 34 (52.3) |
| Female | 86 (43.4) | 73 (50.7) | 34 (50.0) | 34 (45.9) | 5 (25.0) | 9 (56.3) | 9 (40.9) | 9 (47.4) | 4 (44.4) | 24 (45.3) | 31 (47.7) |
| Diagnosis | |||||||||||
| MF | 134 (67.7) | 105 (72.9) | 47 (69.1) | 53 (71.6) | 13 (65.0) | 5 (31.2) | 16 (72.7) | 14 (73.7) | 5 (55.6) | 10 (18.9) | 48 (73.8) |
| SS | 64 (32.3) | 39 (27.1) | 21 (30.9) | 21 (28.4) | 7 (35.0) | 11 (68.8) | 6 (27.3) | 5 (26.3) | 4 (44.4) | 43 (81.1) | 17 (26.2) |
| Stage at diagnosis | |||||||||||
| Early | 93 (43.0) | 66 (45.8) | 32 (47.1) | 35 (47.3) | 11 (55.0) | 2 (12.5) | 13 (59.1) | 5 (26.3) | 2 (22.2) | 3 (5.7) | 37 (56.9) |
| Advanced | 105 (57.0) | 78 (54.2) | 36 (52.9) | 39 (52.7) | 9 (45.0) | 14 (87.5) | 9 (40.9) | 14 (73.7) | 7 (77.8) | 50 (94.3) | 28 (43.1) |
| 1A | 51 (25.8) | 31 (21.5) | 12 (17.6) | 15 (20.3) | 5 (25.0) | 0 (0) | 8 (36.4) | 2 (10.5) | 0 (0) | 1 (1.9) | 14 (21.5) |
| 1B | 41 (20.7) | 35 (24.3) | 20 (29.4) | 19 (25.7) | 6 (30.0) | 2 (12.5) | 5 (22.7) | 3 (15.8) | 2 (22.2) | 2 (3.8) | 23 (35.4) |
| 2A | 1 (0.5) | 0 (0) | 0 (0) | 1 (1.4) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) |
| 2B | 30 (15.2) | 35 (24.3) | 12 (17.7) | 13 (17.6) | 0 (0) | 3 (18.8) | 4 (18.2) | 5 (26.3) | 2 (22.2) | 0 (0) | 11 (16.9) |
| 3A | 6 (3.0) | 2 (1.4) | 3 (4.4) | 3 (4.0) | 1 (5.0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 3 (5.7) | 3 (4.6) |
| 3B | 8 (4.0) | 0 (0) | 3 (4.4) | 1 (1.4) | 1 (5.0) | 1 (6.2) | 0 (0) | 1 (5.3) | 2 (22.2) | 6 (11.3) | 0 (0) |
| 4A | 58 (29.3) | 38 (26.4) | 18 (26.5) | 21 (28.4) | 6 (30.0) | 10 (62.5) | 5 (22.7) | 6 (31.6) | 3 (33.3) | 41 (77.3) | 13 (20.0) |
| 4B | 3 (1.5) | 3 (2.1) | 0 (0) | 1 (1.4) | 1 (5.0) | 0 (0) | 0 (0) | 2 (10.5) | 0 (0) | 0 (0) | 1 (1.5) |
| Folliculotropic | |||||||||||
| Non-FT | 185 (93.5) | 141 (97.9) | 60 (88.2) | 66 (89.2) | 19 (95.0) | 16 (100) | 21 (95.5) | 17 (89.5) | 8 (88.9) | 53 (100) | 61 (93.8) |
| FT | 13 (6.5) | 3 (2.1) | 8 (11.8) | 8 (10.8) | 1 (5.0) | 0(0) | 1 (4.5) | 2 (10.5) | 1 (11.1) | 0 (0) | 4 (6.2) |
| Tumor (skin) score at treatment commencement | |||||||||||
| T1 | 33 (4.7) | 3 (2.2) | 5 (7.3) | 2 (2.7) | 1 (5.0) | 0 (0) | 1 (4.5) | 1 (5.6) | 1 (12.5) | 0 (0) | 2 (3.1) |
| T2 | 244 (35.0) | 40 (28.8) | 31 (45.6) | 24 (32.4) | 9 (45.0) | 2 (12.5) | 8 (36.4) | 4 (22.2) | 2 (25.0) | 6 (11.3) | 27 (42.2) |
| T3 | 168 (24.1) | 53 (38.1) | 11 (16.2) | 21 (28.4) | 2 (10.0) | 2 (12.5) | 5 (22.7) | 8 (44.4) | 3 (37.5) | 0 (0) | 20 (31.3) |
| T4 | 252 (36.2) | 43 (30.9) | 21 (30.9) | 27 (36.5) | 8 (40.0) | 12 (75.0) | 8 (36.4) | 5 (27.8) | 2 (25.0) | 47 (88.7) | 15 (23.4) |
| Nodal involvement at treatment commencement | |||||||||||
| N0 | 487 (69.9) | 82 (60.3) | 51 (77.3) | 52 (70.3) | 14 (70.0) | 9 (56.2) | 16 (76.2) | 7 (41.2) | 7 (87.5) | 35 (66.0) | 53 (82.8) |
| N1, N2, N3, Nx | 210 (30.1) | 54 (39.7) | 15 (22.7) | 22 (29.7) | 6 (30.0) | 7 (43.8) | 5 (23.8) | 10 (58.8) | 1 (12.5) | 18 (34.0) | 11 (17.2) |
| Visceral involvement at treatment commencement | |||||||||||
| M0 | 680 (97.4) | 133 (95.7) | 68 (100) | 73 (98.7) | 19 (95.0) | 16 (100) | 21 (95.5) | 14 (77.8) | 8 (100) | 52 (98.1) | 63 (96.9) |
| M1 | 18 (2.6) | 6 (4.3) | 0 (0) | 1 (1.3) | 1 (5.0) | 0 (0) | 1 (4.5) | 4 (22.2) | 0 (0) | 1 (1.9) | 2 (3.1) |
| Blood involvement at treatment commencement | |||||||||||
| B0 | 484 (69.4) | 102 (73.4) | 49 (72.1) | 54 (73.0) | 16 (80.0) | 7 (43.8) | 18 (81.8) | 11 (61.1) | 5 (62.5) | 10 (18.9) | 52 (80.0) |
| B1 | 38 (5.5) | 1 (0.7) | 3 (4.4) | 5 (6.7) | 0 (0) | 0 (0) | 1 (4.6) | 2 (11.1) | 1 (12.5) | 8 (15.1) | 4 (6.1) |
| B2 | 175 (25.1) | 36 (25.9) | 16 (23.5) | 15 (20.3) | 4 (20.0) | 9 (56.2) | 3 (13.6) | 5 (27.8) | 2 (25.0) | 35 (66.0) | 9 (13.9) |
| LCT at treatment commencement | |||||||||||
| Non-LCT | 615 (86.7) | 106 (73.6) | 64 (94.1) | 60 (81.1) | 18 (90.0) | 16 (100) | 20 (90.9) | 11 (57.9) | 8 (88.9) | 53 (100) | 62 (95.4) |
| LCT | 94 (13.3) | 38 (26.4) | 4 (5.9) | 14 (18.9) | 2 (10.0) | 0 (0) | 2 (9.1) | 8 (42.1) | 1 (11.1) | 0 (0) | 3 (4.6) |
| Median age at diagnosis, y | 61.8 | 56.8 | 57.8 | 58.0 | 64.6 | 57.7 | 60.7 | 58.9 | 40.6 | 64.7 | 67.4 |
| Median age at treatment, y | 62.5 | 62.6 | 60.3 | 63.4 | 66.6 | 61.5 | 62.9 | 59.9 | 42.1 | 65.8 | 67.7 |
| Median treatment line (range) | 3 (1-13) | 4 (1-12) | 2 (1-9) | 3 (1-9) | 2 (1-11) | 3.5 (1-8) | 4 (1-11) | 3 (1-11) | 6 (2-10) | 2 (1-11) | 2 (1-8) |
Early stage includes IA-IIA. Advanced stage includes IIB-IVB. TNMB scores are as defined by the WHO-EORTC revised staging criteria. All values are presented as n (%) unless otherwise noted.
FT, folliculotropic; LCT, large-cell transformation; SCT, stem cell transplant; TSEB, total skin electron beam.
The median number of systemic treatments administered to each patient was 3 (range 1-13), resulting in a total of 709 episodes of treatment available for analysis. PUVA was the most common first-line therapy overall (n = 37; 23.7%) and used most frequently in patients with early-stage disease (35.5%). In patients with advanced-stage disease, low-dose methotrexate was the most prevalent first-line therapy used (21.9%; n = 23). Of note, we used ECP as first-line therapy in 29.9% of patients with T4 (erythrodermic) disease.
A small proportion of patients was concurrently receiving a SDT during the course of their systemic therapy; 7.1% of patients received concurrent nbUVB and 6.1% received concurrent local radiotherapy. The concurrent use of topical steroids was very frequent among the patient cohort and not formally documented in the database.
TTNT analysis
Results for treatment groups are summarized in Table 4. The median TTNT for all treatments analyzed together was 5.4 months (95% CI 5.1-6.1). The outcomes were somewhat inferior in patients with transformed disease (n = 94; 13.3%) where overall TTNT was 4.7 months (95% CI 3.2-5.8), compared with 5.6 months (95% CI 5.0-6.4) for nontransformed disease (n = 615; 86.7%) (P = .004). There was no difference identified for those with folliculotropic-variant disease (n = 13) who had an overall TTNT of 5.0 months (95% CI 3.3-7.3; P = .64).
TTNT analysis
| . | Median TTNT (mo) . | TTNT 95% CI (mo) . | 1-y free from further treatment (%) . | 2-y free from further treatment (%) . | Median line of therapy . |
|---|---|---|---|---|---|
| All treatments | 5.4 | 5.1-6.1 | 29.2 | 21.4 | 3 |
| Chemotherapy | 3.9 | 3.2-5.1 | 10.7 | 5.4 | 4 |
| α-interferon | 8.7 | 6.0-18.0 | 41.7 | 29.1 | 2 |
| HDACi | 4.5 | 4.0-6.1 | 20.0 | 14.5 | 3 |
| Bexarotene | 7.3 | 2.6-110.8 | 47.4 | 36.8 | 2 |
| Alemtuzumab | 4.1 | 2.7-6.5 | 27.8 | 27.8 | 3.5 |
| Denileukin diftitox | 5.1 | 2.7-6.5 | 22.7 | 22.7 | 4 |
| AuSCT | 7.8 | 4.7-24.4 | 41.5 | 28.4 | 3 |
| AlloSCT | 34.6 | 11.5-N/A | 80.0 | 53.3 | 6 |
| ECP | 9.2 | 5.9-12.8 | 39.1 | 25.7 | 2 |
| TSEB | 7.8 | 4.4-14.7 | 39.0 | 26.5 | 2 |
| Low-dose methotrexate | 5.0 | 3.6-6.5 | 25.1 | 21.2 | 2 |
| . | Median TTNT (mo) . | TTNT 95% CI (mo) . | 1-y free from further treatment (%) . | 2-y free from further treatment (%) . | Median line of therapy . |
|---|---|---|---|---|---|
| All treatments | 5.4 | 5.1-6.1 | 29.2 | 21.4 | 3 |
| Chemotherapy | 3.9 | 3.2-5.1 | 10.7 | 5.4 | 4 |
| α-interferon | 8.7 | 6.0-18.0 | 41.7 | 29.1 | 2 |
| HDACi | 4.5 | 4.0-6.1 | 20.0 | 14.5 | 3 |
| Bexarotene | 7.3 | 2.6-110.8 | 47.4 | 36.8 | 2 |
| Alemtuzumab | 4.1 | 2.7-6.5 | 27.8 | 27.8 | 3.5 |
| Denileukin diftitox | 5.1 | 2.7-6.5 | 22.7 | 22.7 | 4 |
| AuSCT | 7.8 | 4.7-24.4 | 41.5 | 28.4 | 3 |
| AlloSCT | 34.6 | 11.5-N/A | 80.0 | 53.3 | 6 |
| ECP | 9.2 | 5.9-12.8 | 39.1 | 25.7 | 2 |
| TSEB | 7.8 | 4.4-14.7 | 39.0 | 26.5 | 2 |
| Low-dose methotrexate | 5.0 | 3.6-6.5 | 25.1 | 21.2 | 2 |
AlloSCT, allogeneic hematopoietic SCT; N/A, not able to be assessed; TSEB, total skin electron beam radiation.
PUVA.
PUVA was used as first-line therapy in 75.8% of patients (median treatment line = 1; range 1-7) and was associated with the longest TTNT, with a median of 36.3 months (95% CI 9.5-47.9). At 2 years, 54.2% of these patients still had not required further treatment. These patients often had early-stage disease, with 50 patients (82.0%) considered T1 or T2 at the start of therapy.
Chemotherapy.
Single-agent or multiagent chemotherapy provided a median TTNT of 3.9 months (95% CI 3.2-5.1) (Table 2 and Figure 1). At 1 year, only 10.7% of patients who received chemotherapy had not progressed to receiving subsequent treatment. Chemotherapy was used generally as second-line therapy (median = 2; range 1-12). The median age at commencement of treatment was 62.6 years. CHOP-like chemotherapy regimens gave the longest TTNT, with a median 5.7 months, and fludarabine-based chemotherapy gave the shortest, with a median 2.2 months. High-dose methotrexate (not included in amalgamated chemotherapy analyses) gave a short TTNT of 2.1 months only (95% CI 1.6-3.2).
TTNT for chemotherapy across treatment lines. Patients receiving chemotherapy as first-line, mid-line (2nd-4th line), or late-line (5th line and later) therapy had significantly different TTNT. The longest TTNT was with first-line use.
TTNT for chemotherapy across treatment lines. Patients receiving chemotherapy as first-line, mid-line (2nd-4th line), or late-line (5th line and later) therapy had significantly different TTNT. The longest TTNT was with first-line use.
Median TTNT when chemotherapy was used as first-line treatment was 7.9 months; however, only 17 of the total 144 patients had chemotherapy at first-line treatment. As mid-line treatment (n = 64), TTNT was 4.8 months and 92.1% of patients had received further treatment within 1 year. As late-line treatment (n = 63), median TTNT for chemotherapy was just 3.2 months, with 93.2% of patients requiring further treatment within 1 year.
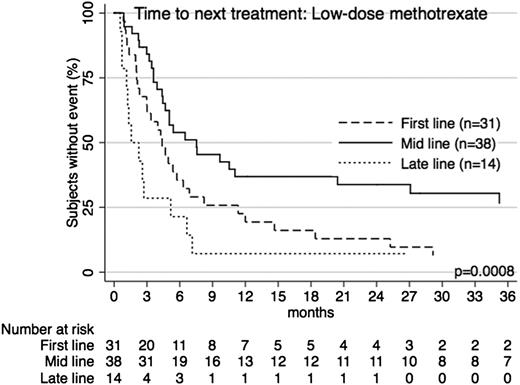
Low-dose methotrexate.
Low-dose methotrexate was frequently used (n = 84), particularly as an early treatment option, with median use at second line (range 1-10). Indeed it was the most common first-line option overall among patients with advanced-stage disease (n = 23; 21.9%). Approximately 58.3% of patients had advanced-stage disease at diagnosis, and the median TTNT was 5.0 months (95% CI 3.6-6.5), with 25.1% of patients free from further treatment at 1 year. When used as a first-line option, median TTNT was 4.4 months (95% CI 2.1-11.3). At mid-line, median TTNT was 7.5 months (95% CI 3.6-36.5) and at late-line was 1.6 months (95% CI 1.1-5.2) (Figure 2). The median age at treatment of these patients was 66.2 years.
TTNT for low-dose methotrexate across treatment lines. Patients receiving low-dose methotrexate as first-line, mid-line (2nd-4th line), or late-line (5th line and later) therapy had significantly different TTNT (P = .0008). The longest TTNT was with mid-line use.
TTNT for low-dose methotrexate across treatment lines. Patients receiving low-dose methotrexate as first-line, mid-line (2nd-4th line), or late-line (5th line and later) therapy had significantly different TTNT (P = .0008). The longest TTNT was with mid-line use.
Biological response modifiers.
With respect to nonchemotherapy agents, α-interferon gave a median TTNT of 8.7 months (95% CI 6.0-18.0), with 41.7% of patients free from further treatment at 1 year. α-interferon was used in patients with a median age at treatment of 60.3 years and was generally used as an early treatment option, with median use as second-line therapy (range 1-9).
HDACi provided a median TTNT of 4.5 months (95% CI 4.0-6.1). At 1 year, 20.0% of patients had not yet had further treatment. HDACi patients were a median age of 63.4 years at the start of treatment. HDACi were generally used as third-line treatment (median = 3, range 1-9).
Bexarotene was used in a small number of patients (n = 20) because of lack of regulatory authority reimbursement. It showed a TTNT of 4.1 months (95% CI 2.7-110.8). At 1 year, 47.4% of patients had not yet had a subsequent treatment. Bexarotene was frequently used as early-line treatment, with median use as second-line treatment (range 1-11), in patients a median age of 66.6 years at commencement of drug.
Alemtuzumab was used in 16 patients and gave a median TTNT of 4.1 months. At 1 year, 27.8% of patients were free from further treatment, and this number was unchanged at 2 years. The majority (87.5%; n = 14) of these patients had advanced-stage disease and 75% (n = 12) were T4 at commencement of alemtuzumab. Alemtuzumab had a median treatment line use of 3.5 (range 1-8) and the median age at commencement of drug was 61.5 years.
Denileukin diftitox (n = 22) provided a median TTNT of 5.1 months and at 1 year 22.7% of patients had not yet had further treatment. Of those who reached 1 year without a subsequent treatment, none required a new treatment in the year after this. The median treatment line was 4 (range 1-11) and age at treatment commencement was 62.9 years. Approximately 59.1% of denileukin patients had early-stage disease at diagnosis, though at commencement of treatment 59.1% were considered T3 or T4.
Allogeneic transplant.
AlloSCT (n = 9) was used as a median sixth-line therapy (range 2-10), yet provided a median TTNT of 34.6 months, with 80% of patients not requiring further treatment at 1 year. Compared with all other treatment groups, these patients were the youngest at diagnosis (median = 40.6 years) and at treatment commencement (median = 42.1 years).
Autologous transplant.
AuSCT was used in 19 patients and provided a median TTNT of 7.8 months. At 1 year, 41.5% of patients remained free from further treatment. AuSCT patients primarily had advanced-stage disease at diagnosis (73.7%), and a large proportion had transformed disease before the commencement of treatment (n = 8; 42.1%). At the commencement of treatment, these patients were more likely to have nodal (58.8%) and/or visceral disease (22.2%). AuSCT was used as median third-line treatment (range 1-11) in patients with a median age of 59.9 years at transplant.
ECP.
ECP was predominantly used in patients with T4 disease (n = 47; 88.7%). Patients were a median age of 65.8 years at treatment, and ECP was generally used as second-line therapy (range 1-11). ECP provided 9.2 months TTNT, with 39.1% requiring no further treatment at 1 year.
Comparisons of TTNT: chemotherapy vs α-interferon
When compared directly with chemotherapy, α-interferon provided a significantly longer TTNT (P < .00001) (Figure 3). With respect to the effect of disease stage, α-interferon TTNT remained significantly longer in those with early-stage at diagnosis (P < .00001) and advanced-stage at diagnosis (P = .0002), and those who had a diagnosis of SS (P = .0027) or MF (P < .00001). At the commencement of therapy, there was a difference in the groups with respect to T-score at commencement of treatment (P = .003): 69% of chemotherapy patients were T3 or T4 at commencement of treatment compared with 47% of α-interferon patients. Nonetheless, when comparisons were made within these groups, T1, T2, and T4 patients all had longer TTNT on α-interferon (P = .0216, P = .0015, and P = .0013, respectively) than chemotherapy. Patients who were T3 at commencement of treatment had no significant difference in TTNT (P = .2098). Age at treatment did not influence the superior efficacy of interferon (<60 years, P = .0238; ≥60 years, P < .00001).
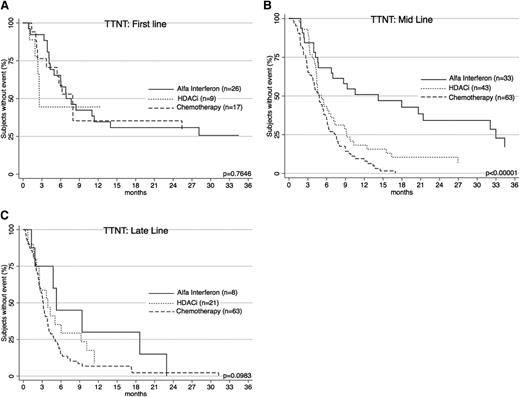
TTNT for α-interferon, HDACi, and chemotherapy. TTNT for these treatment groups were significantly different (P < .00001). α-interferon gave the longest TTNT. Both α-interferon and HDACi had significantly longer TTNT than chemotherapy.
TTNT for α-interferon, HDACi, and chemotherapy. TTNT for these treatment groups were significantly different (P < .00001). α-interferon gave the longest TTNT. Both α-interferon and HDACi had significantly longer TTNT than chemotherapy.
Interesting differences in TTNT are noted when treatments are compared for line of therapy (Figure 4). Interferon was generally used earlier (2nd-line vs 4th-line for chemotherapy; P < .0001). To account for this, patients were separately analyzed according to line of therapy. When used as a first-line treatment, there was no difference in TTNT between chemotherapy and α-interferon (P = .9836). When used as mid-line treatment, interferon performed significantly better (P < .00001). As a late-line therapy, there was a nonsignificant trend in favor of interferon (P = .0848). Importantly, when comparing chemotherapy and interferon cohorts, there was no significant difference with respect to age at diagnosis (P = .8), age at treatment commencement (P = .5), gender (P = .9), diagnosis (P = .6), or proportion of early- and advanced-stage patients (P = .9).
TTNT for α-interferon, HDACi, and chemotherapy across treatment lines. (A) TTNT at first line for the 3 therapies is shown. There is no significant difference in efficacy. (B) TTNT at mid line (2nd-4th line) shows α-interferon is the best performer. (C) TTNT at late line (5th line and later) shows a trend toward α-interferon.
TTNT for α-interferon, HDACi, and chemotherapy across treatment lines. (A) TTNT at first line for the 3 therapies is shown. There is no significant difference in efficacy. (B) TTNT at mid line (2nd-4th line) shows α-interferon is the best performer. (C) TTNT at late line (5th line and later) shows a trend toward α-interferon.
Comparisons of TTNT: chemotherapy vs HDACi
When compared directly with chemotherapy, HDACi provided a significantly longer TTNT (4.5 vs 3.9 months; P = .0111) (Figure 3). The patient populations had similar characteristics with respect to age at diagnosis (P = .5516), age at treatment (P = .6255), gender (P = .507), diagnosis (MF vs SS) (P = .461), stage (P = .461), T-score (P = .562), transformation status (P = .220), or line of therapy used (P = .083). HDACi were superior for patients with advanced-stage disease (P = .0296) and those who were T4 (erythrodermic) at commencement of treatment (P = .0017). Efficacy was equivalent in patients with early-stage disease (P = .1338), T1 (P = .0634), T2 (P = .9449), and T3 (P = .6748). When used as a first-line or late-line therapy, HDACi and chemotherapy performed similarly (P = .4823 and P = .1324, respectively) (Figure 4). When used as a mid-line therapy, HDACi gave a significantly longer TTNT (P = .0325). For patients aged 60 and above, HDACi had a significantly better TTNT (P = .0101).
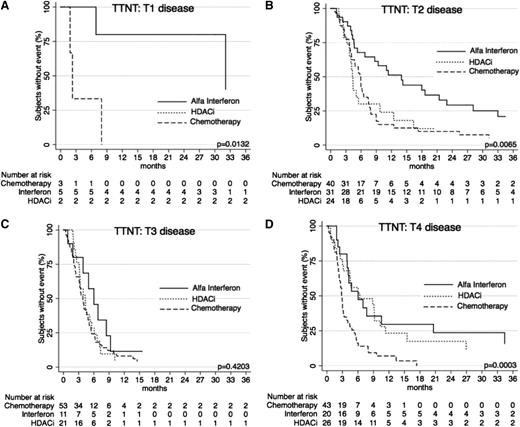
Comparisons of TTNT: stratification by stage and T-score
When further stratified by T (skin) score at commencement of treatment, TTNT curves show similar results (Figures 5 and 6) for the systemic therapies of α-interferon, HDACi, and chemotherapy, with α-interferon and HDACi providing a longer TTNT than chemotherapy for T1, T2, and T4 disease (P = .0132, P = .0065, and P = .0003, respectively), and equivalent in T3 disease (P = .4203). Similarly, when these therapies were compared using clinical stage, results again demonstrated that chemotherapy provided the shortest TTNT for IA-IIA disease (P = .0002), IIB disease (P = .0479), and IVA-IVB disease (P = .0023). Of note in IIIA-IIIB disease, outcomes were not significantly different (P = .8427).
TTNT for α-interferon, HDACi, and chemotherapy by T (skin) score. (A) TTNT in patients with T1 disease for the 3 key therapies. Note: There are only 2 points on the graph for HDACi, so results are not visible as a curve. (B) TTNT in patients with T2 disease for the 3 therapies. (C) TTNT for patients for the 3 therapies. (D) TTNT for patients with T4 disease for the 3 therapies.
TTNT for α-interferon, HDACi, and chemotherapy by T (skin) score. (A) TTNT in patients with T1 disease for the 3 key therapies. Note: There are only 2 points on the graph for HDACi, so results are not visible as a curve. (B) TTNT in patients with T2 disease for the 3 therapies. (C) TTNT for patients for the 3 therapies. (D) TTNT for patients with T4 disease for the 3 therapies.
TTNT for α-interferon, HDACi, and chemotherapy by stage. (A) TTNT in patients with stage IA, IB, and IIA disease for the 3 therapies. (B) TTNT in patients with stage IIB disease for the 3 therapies. (C) TTNT for patients with stage III disease for the 3 therapies. (D) TTNT for patients with stage IVA and IVB disease for the 3 therapies.
TTNT for α-interferon, HDACi, and chemotherapy by stage. (A) TTNT in patients with stage IA, IB, and IIA disease for the 3 therapies. (B) TTNT in patients with stage IIB disease for the 3 therapies. (C) TTNT for patients with stage III disease for the 3 therapies. (D) TTNT for patients with stage IVA and IVB disease for the 3 therapies.
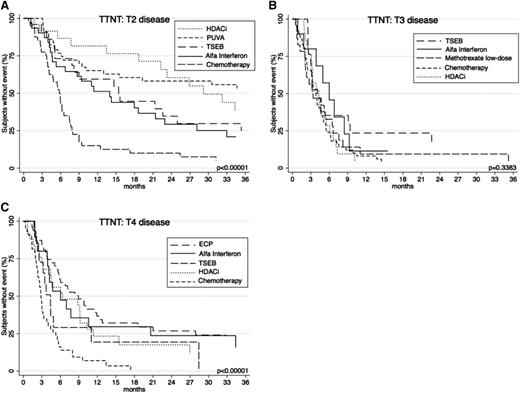
For completeness, a broader range of the common systemic therapies used at each level of skin-burden disease was subsequently compared (Figure 7). In patients with T2 disease, TTNT was comparatively assessed among key therapies HDACi, PUVA, TSEB, α-interferon, and chemotherapy. Analysis revealed that HDACi and PUVA provided the longest TTNT (P < .00001) compared with others. In patients with T3 disease, differences between α-interferon, TSEB, methotrexate low-dose, HDACi, and chemotherapy showed a nonsignificant difference in TTNT (P = .3383). In patients with T4 disease, the longest TTNT was seen with ECP and α-interferon (P < .00001) when compared with other treatments HDACi, TSEB, and chemotherapy.
TTNT for key therapies according to T (skin) score. (A) TTNT for PUVA, α-interferon, HDACi, TSEB, and chemotherapy in patients with T2 disease. (B) TTNT for α-interferon, HDACi, chemotherapy, TSEB, and low-dose methotrexate in patients with T3 disease. (C) TTNT for α-interferon, HDACi, chemotherapy, ECP, and TSEB in patients with patients with T4 disease.
TTNT for key therapies according to T (skin) score. (A) TTNT for PUVA, α-interferon, HDACi, TSEB, and chemotherapy in patients with T2 disease. (B) TTNT for α-interferon, HDACi, chemotherapy, TSEB, and low-dose methotrexate in patients with T3 disease. (C) TTNT for α-interferon, HDACi, chemotherapy, ECP, and TSEB in patients with patients with T4 disease.
Discussion
In recent years, with the increasing interest in the role of biological agents in T-cell lymphomas, there have been moderately large studies examining the role of vorinostat, romidepsin, panobinostat, pralatrexate, denileukin diftitox, and lenalidomide in MF/SS.24,30-34,40,53 There are also published studies of single-agent and combination chemotherapy regimens for MF/SS (Table 1). Surprisingly, there are no studies comparing conventional chemotherapy with biological agents in these disease groups.
We sought to undertake a retrospective analysis of a large group of MF/SS patients requiring systemic therapy. We recognize the deficiencies of such a retrospective study in addition to the idiosyncrasies of local drug availability such as in the cases of bexarotene, pralatrexate, and denileukin diftitox, where lack of regulatory authority reimbursement means drugs are generally not available outside of clinical trials. Nonetheless, individual treatment groups had generally large sample sizes such that CIs were narrow for many subgroup analyses. We chose a clinically meaningful and objective end point, TTNT, although we recognize that there remains the subjectivity of the treating clinician; the decision to intervene with a subsequent therapy is dependent not only on the patient’s pattern of disease but also upon the clinician’s intuitive concern of prognosis, as well as the availability, toxicities, and schedules of the treatments themselves. We report the results of 709 treatment episodes in 198 patients with MF/SS.
Overall, our analysis highlights the inability of chemotherapy to provide durable disease control. Chemotherapy offered only a short median TTNT of 3.9 months. There was no plateau on the curve, with 89% of patients requiring further treatment within 1 year. In this study, we did not evaluate RR as a major end point, primarily because of the changes in assessment of response criteria over the timeframe of this analysis and the inability to apply the modern mSWAT-based criteria in a retrospective fashion. Nonetheless, prior studies of individual chemotherapy regimens (Table 1) have generally demonstrated respectable RR, but durable remissions are rare. We were unable to identify any 1 optimal chemotherapy regimen. Of note, we failed to establish a superior efficacy with gemcitabine-based chemotherapy, as has been suggested in prior studies.18-20 TTNT for gemcitabine-based therapy was not found to be significantly different from other modalities in this cohort (Table 2).
Chemotherapy was most efficacious when used as first-line therapy, but efficacy quickly declined when it was used as mid- or late-line therapy, non–first-line therapy being where the majority of patients in this study underwent chemotherapy.
Low-dose methotrexate gave a TTNT of 5 months, despite its frequent use in patients with advanced-stage disease. These promising results suggest that low-dose methotrexate should be considered as an effective alternative to more toxic multiagent chemotherapy regimens. Our study also demonstrated the relative efficacy of other biological agents including denileukin diftitox, bexarotene, and alemtuzumab.
Although the best outcome for chemotherapy was at first-line use, TTNT was still not significantly better than that achieved with α-interferon or HDACi used at any treatment line. Indeed α-interferon and HDACi both provided significantly greater TTNT overall compared with chemotherapy. We believe comparisons of efficacy between these treatment groups are valid, demonstrated by nonsignificant differences in key characteristics of the patient populations for each treatment, including age, gender, and stage at diagnosis. Further, patients treated with HDACi or chemotherapy were particularly similar, with nonsignificant differences in T-score (skin burden) at commencement of treatment and in treatment line use. With respect to α-interferon, we observed that chemotherapy patients had generally higher T-scores, and treatment was used as later-line therapy, reflective of current treatment guidelines.9-11,13 Thus to more accurately compare efficacy, we analyzed T subgroups separately and confirmed that interferon performed better in all skin stages of disease, except for tumor-stage (T3) disease, where differences in efficacy were nonsignificant. Importantly, we found that that interferon performs better than chemotherapy as mid- or late-line therapy and is equivalent to chemotherapy when used at first line.
Although sample size was small (n = 9), this study showed that AlloSCT resulted in excellent disease control in selected patients. These patients were much younger than typical MF/SS patients and predominantly had advanced-stage disease. AlloSCT provided a durable response, with only 20% requiring further treatment within 1 year. In appropriate patients, AlloSCT may be the best therapeutic option for an effective and durable remission. Indeed, akin to this strategy used in T-cell prolymphocytic leukemia,54 we are exploring the role of initial biological control with alemtuzumab followed by planned AlloSCT, particularly in patients with SS.
This analysis demonstrates the limited durability of disease control with chemotherapy. Adverse effects, particularly the risk of infection, vary greatly with each therapy. Drugs such as forodesine have known T-cell effects,55 but even novel agents can increase the risk of infection.56-58 Data on adverse events was not available for this patient cohort, but it remains important for the clinician to consider the balance of these in guiding management decisions. Taken together, we recommend against the routine use of chemotherapy in patients with MF/SS and strongly propose that chemotherapy be reserved for use after the optimum utilization of biological therapies.
The online version of this article contains a data supplement.
There is an Inside Blood Commentary on this article in this issue.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank the University of Melbourne Medical School for their support.
C.F.M.H. was a Doctor of Medicine student within the department in 2013-2014.
Authorship
Contribution: C.F.M.H. collected and analyzed data, performed statistical analysis, and wrote the manuscript; H.M.P. designed the research and wrote the manuscript; A.K. designed research and provided support; R.T. collected data and maintained the database; C.W. formatted data and provided statistical guidance; and C.M., S.L., D.A.W., O.B., K.N., C.T., M.D., G.R., and D.R. provided critical input to intellectual content.
Conflict-of-interest disclosure: H.M.P. has received research grants and honorarium from Celgene Corporation, Novartis, and Merck. M.D. has received research grants and honorarium from Celgene Corporation and Novartis. D.R. has received research grants and honorarium from Celgene Corporation and Novartis. D.A.W. has received research grants from Celgene Corporation. The remaining authors declare no competing financial interests.
Correspondence: Professor H. Miles Prince, Division of Cancer Medicine, Peter MacCallum Cancer Centre, Locked Bag 1, A’Beckett St, Melbourne, Victoria, Australia 8006; e-mail: miles.prince@petermac.org.