In this issue of Blood, Wang et al demonstrate how mutations in the Wnt/β-catenin pathway in chronic lymphocytic leukemia (CLL) lead to activation of this pathway.1 The work presented demonstrates that this pathway is likely to contribute to CLL pathogenesis, at least in a subset of patients, and suggests that it may contain potential therapeutic targets. These observations are following the authors' groundbreaking work on the genetic landscape in CLL2,3 and by using exciting, novel transfection technology (ie, silicon nanowires).4 Thus, the notoriously difficult-to-transfect CLL cells could be modified to be able to target a pathway that has been identified by indirect evidence from next generation sequencing, demonstrating that attacking the mutated proteins by genetic modification is feasible. Indeed, at least for some proteins tested, modification of the pathway on a number of levels led to changes in CLL behavior consistent with a role of the Wnt/β-catenin pathway in CLL pathogenesis. The paper must be read to fully appreciate the elegance of the approaches taken, as well as understand the potential limitations of the approach.
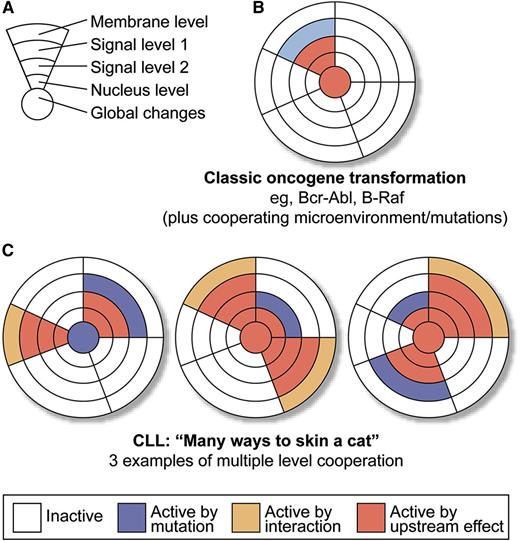
Transformation via “canonical hits” vs “pathway tweaking.” (A) Schematic depiction of a generic pathway and possible levels of “oncogenic” lesions: “membrane level” includes extracellular signals, both from the microenvironment (including BCR signaling) or via autocrine stimulation, as well as mutations in receptors; “signaling levels 1 and 2” simplify different levels of action in a single pathway; “nuclear controls” mean transcription factor changes; and “global controls” mean changes in micro RNA, epigenetics, splicing machinery, or DNA damage response. (B) Model of classic oncogene transformation (1 strong and highly recurrent driver leads to clonal expansion). Of course, this is a gross simplification, whereas in reality, there are collaborating lesions in all these instances. (C) Multiple ways of transformation in CLL by activation with or without mutation, and on multiple levels of a defined set of collaborating pathways. Professional illustration by XavierStudio.
Transformation via “canonical hits” vs “pathway tweaking.” (A) Schematic depiction of a generic pathway and possible levels of “oncogenic” lesions: “membrane level” includes extracellular signals, both from the microenvironment (including BCR signaling) or via autocrine stimulation, as well as mutations in receptors; “signaling levels 1 and 2” simplify different levels of action in a single pathway; “nuclear controls” mean transcription factor changes; and “global controls” mean changes in micro RNA, epigenetics, splicing machinery, or DNA damage response. (B) Model of classic oncogene transformation (1 strong and highly recurrent driver leads to clonal expansion). Of course, this is a gross simplification, whereas in reality, there are collaborating lesions in all these instances. (C) Multiple ways of transformation in CLL by activation with or without mutation, and on multiple levels of a defined set of collaborating pathways. Professional illustration by XavierStudio.
The paper has impact beyond CLL that is worth exploring. First, the authors attack a recurrent problem, observed during the recent explosion of genetic knowledge on tumorigenesis. Massively parallel sequencing approaches suggest that certain mutations in tumors act as so-called “drivers” largely using a “guilt-by-association” approach. The logic is that recurrent mutations selected by independent tumor evolution pathways must be important. If the mutation can be also placed into the context of a plausible oncogenic pathway, then we tend to believe that we have, indeed, found the culprits. We commend Wang et al1 for taking their own observations from genetics to a cell biology platform and their attempts to move a step closer to elucidating not just the association, but the action of the mutations they described earlier.
Of course, such approaches have been taken in the past, but mainly for a limited type of lesion, leading to the second point. We, as hematologists (I cannot speak for all hematologists, but as a figure of speech, allow me to say) have been raised on simple tumor biology models, not the least because it was in hematology, where some of the first insights into molecular oncogenesis were developed. Single and highly recurrent lesions, such as the Bcr-Abl or PML-RARA translocations (or more recently B-Raf mutations) were identified, validated in cell biology and animal models to the point where it was possible to stringently identify culprits, fulfilling Koch’s postulates, and targeted treatment could be initiated effectively. This enormous success story may have biased our views and expectations in many malignancies, until the arrival of next-generation sequencing and the confusing complexity we have “endured” since then. Enter CLL! CLL seems a very particular beast in that is a prototypically odd malignancy for a number of reasons. First, it shows an intricate interaction and dependency on multiple microenvironmental cues (see the review by Burger5 ), suggesting that not all transforming “events” need to be hardwired by mutations in the leukemic clone itself. Indeed, CLL induces signaling in its microenvironment that seems essential for its survival.6 Second, with the possible exception of deletion 13q, no real high frequency transforming events have been defined yet. Indeed, the genetic landscape, as previously demonstrated by the authors of this paper in discussion, looks extraordinarily “colorful.”2,3 The important achievement of the Dana Farber group was to use a bioinformatic pattern recognition approach (potentially flawed by biases) to align the mutations with core pathways, seemingly mutated in CLL recurrently, with a higher frequency than the individual mutations suggested. The importance of this paper is to create a dataset that corroborates the idea that the genetic activation or silencing of a pathway can (and will) occur on multiple levels, with a relatively consistent effect on the output of the overall pathway. To phrase this on a more general level, as opposed to the classic paradigm of a recurrent mutation in a defined oncogene or tumor suppressor as a hallmark event in transformation (panels A and B) a picture of a much more promiscuous pattern of transformation emerges for CLL (and as a paradigm for a number of other malignancies). There seems to be a set of defined evolutionary “tasks” that a CLL clone has to “take care of” (ie, a combination of activated pathways that needs to be present in the cell for effective transformation). In contrast to the simpler classical model, it seems that CLL is not very restricted regarding the type of “solutions” emerging in its evolution. An extracellular (microenvironmental or autocrine) signal may be substituted by a pathway mutation that can happen on multiple levels along the pathway (panel C). Ultimately, the combination of multiple events leads to a progressive CLL clone with the clinical consequences we observe. I would like to stress that these do not necessarily need to be strong biological drivers, individually. Thus, an enormous plethora of solutions may exist for the question: “What is necessary to program a CLL cell?” Although this is not particularly new (ie, Hanahan and Weinberg’s7 seminal Hallmarks of Cancer Perspective), it seems that CLL is particularly “creative” regarding its solutions and Wang et al1 have provided important work on the path to recognizing this.2,3 Finally, although the creativity of a malignancy is generally not our friend, if we are clinicians aiming to treat a disease, the paradigm of activated, and maybe critical, pathways can be exploited to our benefit. Understanding the pathway map that is important to a disease and identifying important nodules (even if, or specifically when, they are not mutated) will lead to successful targets, as can be witnessed currently in the clinical development of BCR signaling inhibitors. Importantly, as Wang et al1 demonstrate that the mutations observed in CLL may be used to “paint” the pathways, we need to target the best effects. Also, the mutations “painted” some really novel scenery. It is exciting times!
Conflict-of-interest disclosure: The author declares no competing financial interests.