Key Points
Upper limb PTS in children depends on DVT pathogenesis (primary vs secondary) and on the age of the patient (neonates vs non-neonates).
DVT pathogenesis and thrombus resolution are independent predictors of upper limb PTS in children.
Abstract
Despite its relatively estimated high occurrence, the characterization of pediatric upper extremity deep vein thrombosis (UE-DVT) and of UE postthrombotic syndrome (PTS) is still lacking. We investigated the occurrence, characteristics, and predictors of UE-PTS in a cohort of children with objectively confirmed UE-DVT. Patients were analyzed in 3 groups according to DVT pathogenesis and neonatal status: primary (G1), secondary neonates (G2neonates), and non-neonates (G2non-neonates). A total of 158 children (23 G1, 25 G2neonates, and 110 G2non-neonates) were included. The most common triggering factors were effort-related (87%) in G1 and central lines in G2neonates (100%) and in G2non-neonates (92%). PTS scores ≥1, as per the Modified Villalta Scale, were identified in 87% of primary patients, 16% of G2neonates, and 49% of G2non-neonates. Survival analysis showed that the time to PTS score ≥1 significantly differed among group (log-rank test P < .0001). A multivariable logistic regression showed that DVT pathogenesis and imaging-determined degree of thrombus resolution at the end of therapy were independent predictors of a PTS score ≥2. In conclusion, pediatric UE-PTS frequency and severity depend on UE-DVT pathogenesis (primary/secondary) and, within the secondary group, on patient’s age. Line-related UE-PTS has a more benign course, particularly in neonates.
Introduction
Approximately 90% to 95% of deep vein thrombosis (DVT) occurring in adult patients affects the lower limbs, whereas only 5% to 10% affects the upper extremities (UE).1 Not surprisingly, because of the relatively high incidence of DVT in the lower extremities, considerably less research has been dedicated to study the characteristics and outcomes of UE-DVT.
Postthrombotic syndrome (PTS), one of the potential outcomes of DVT, is defined as the “chronic venous symptoms and/or signs secondary to DVT and its sequelae.”2,3 UE-PTS has been estimated to occur in approximately 15% of adult patients affected by UE-DVT.4 To date, the existence of residual thrombosis is the only significant predictor for UE-PTS development in this population, whereas axillary and subclavian vein involvement appear to be potential risk factors.5
In contrast to adult patients, 30% to 50% of cases of pediatric DVT involve the UE,6-8 perhaps reflecting the role of central venous lines (CVL) as a risk factor for DVT in the pediatric population. The relatively high frequency of UE-DVT results in a higher expected frequency of UE-PTS in children compared with the adult population, highlighting the need to specifically study this condition and its consequences.
More than a decade ago, the Canadian thrombophilia registry reported 214 pediatric patients affected with UE-DVT, 15 (7%) of whom developed PTS.9 Since then, a few studies have reported data on the long-term outcomes of DVT, and most of them have included thrombotic events involving a wide range of venous territories. These studies have reported a frequency of UE-PTS ranging between 19% and 54%.6-8,10-12
We sought to investigate the specific features of UE-DVT and the incidence and predictors of UE-PTS in a cohort of children followed up at our institution. In addition, we planned to investigate the frequency of thrombus resolution, recurrence, pulmonary embolism (PE), and death related to UE-DVT.
Methods
The health records of children diagnosed with UE-DVT at The Hospital for Sick Children, between January 1999 and July 2011, were retrospectively reviewed. The ethics review board approved this study. Informed consent was waived. This study was conducted in accordance with the Declaration of Helsinki.
Patients who had a thrombotic event objectively confirmed by Doppler ultrasonography, magnetic resonance imaging, computed tomography (CT), and/or venography, and who had at least one PTS assessment no sooner than 6 months after DVT diagnosis were included. In keeping with the institutional protocol, patients who sustain thrombotic events are assessed for PTS in the Pediatric Thrombosis Clinic every 18 months after the end of DVT therapy, until transfer to adult care. Patient care is either carried out or directly supervised by a thrombosis expert. To ensure quality of data, double data entry was performed. The agreement between observers for outcome ascertainment was excellent (weighted κ = 0.85 [95% confidence interval [CI] 0.74-0.95]). A third investigator resolved any disagreements.
Descriptive variables, outcomes, and predictors were defined as follows:
Descriptive variables
1. Patient characteristics and risk factors for UE-DVT included length of follow-up, age at diagnosis of DVT, sex, underlying conditions, use of oral contraceptives (OCP) before sustaining DVT, anthropometric variables, handedness, and major and minor thrombophilia.
Anthropometric variables encompassed body mass index (BMI) or weight-for-length percentile around the time of DVT diagnosis for patients older or younger than 2 years, respectively; BMI was estimated using a SAS macro available through the Centers for Disease Control and Prevention website.13
According to the usual practice at our institution, thrombophilia testing is offered to all patients and it is performed according to the families’ informed decision. Thrombophilia testing was performed and interpreted as described previously.14 Major thrombophilia included protein C, protein S, or antithrombin deficiency, positive lupus anticoagulant or anticardiolipin antibodies as per published criteria,15 and/or factor V Leiden (FVL) or prothrombin gene (PTG) homozygous mutation, or double-heterozygous FVL/PTG mutation. Minor thrombophilia traits included factor VIII or plasma lipoprotein (a) elevation and heterozygous FVL or PTG mutation.
2. Characteristics of the UE-DVT at diagnosis comprised type of thrombotic event (described below), triggering event, laterality, vessel segment and number of segments affected, degree of occlusion, and imaging modality used for diagnosis.
3) Therapy of UE-DVT included type of drug, delay in starting therapy (Δt), dose (full anticoagulation vs prophylaxis16 ), and duration of treatment.
Outcomes
1. The main outcome of the study was PTS determined by the Modified Villalta Scale (MVS), one of the 2 pediatric PTS outcome measure tools accepted by the International Society of Thrombosis and Haemostasis (ISTH) Perinatal and Pediatric Haemostasis Subcommittee.6,17 This tool has been described previously.6,18 Data on signs and symptoms were collected at each visit and then transformed in MVS scores.
2. Resolution of DVT, recurrent DVT, PE, and DVT-related death constituted the secondary outcomes of the study. Resolution of DVT in follow-up imaging was classified as complete, partial, no resolution, or extension, and referred to that documented at end of therapy. End of therapy referred to the time immediately after thrombolysis in patients submitted to such therapy, 3 to 6 months after DVT diagnosis in patients receiving anticoagulation, and 3 months in patients not treated. Recurrent DVT was defined per current ISTH pediatric recommendations.19
Predictors of the outcomes
1. Predictors of PTS: Type of thrombotic event was the main predictor of PTS and the main grouping variable of the study. Thrombotic events were classified as primary and secondary, based on their pathogenesis. Primary UE-DVT comprised idiopathic and effort-related thrombosis, including events related to anatomical variations.20 Secondary UE-DVT included thrombosis triggered by other causes, such as CVL. Patients in the secondary group were further divided according to their age in neonates (0-28 days) and non-neonates (>28 days). Therefore, 3 main groups were compared throughout the study: primary UE-DVT (G1), secondary UE-DVT in neonates (G2neonates), and secondary UE-DVT in non-neonates (G2non-neonates).
Additional PTS predictors comprised age, sex, thrombophilia, symptomatic DVT, vein involved, number of segments affected, degree of occlusion, therapeutic modality and length, Δt, resolution, and recurrence.
2. Predictors of DVT resolution: Predictors included therapeutic modality and thrombophilia.
Statistical analysis
Data analysis was generated using SAS software, version 9.2 (SAS Institute Inc., Cary, NC).
Categorical data were summarized as percentages and ratios. Appropriate measures of central tendency and dispersion were used to report continuous data. Characteristics were compared across groups (G1, G2neonates, and G2non-neonates) using the χ2 test, Fisher exact test, and Mann-Whitney-Wilcoxon U test, as appropriate.
Main outcome.
The frequency of PTS, per the MVS, was compared among groups using the Fisher exact test. Average scores per follow-up periods were estimated. The time to diagnosis of PTS was analyzed in the 3 main groups using the Kaplan-Meier method. Survival curves were compared using the log-rank test.
The probability of having an MVS PTS score of >1 vs ≤1 was modeled using logistic regression. A score >1 was chosen to avoid overcalling PTS, given the retrospective nature of the data. Predictors associated with the outcome at a conservative P value of .20 in simple logistic regression were considered for a multivariable logistic regression model. Multicollinearity was assessed among potential predictors. Model fit was assessed with the Hosmer-Lemeshow test and c-statistics. Influential outliers were investigated. Models were compared using the Akaike Information Criterion (AIC). The final model was chosen on the basis of the clinical significance of the predictors and lowest AIC.
Secondary outcomes.
The incidences of DVT resolution, recurrence, PE, and death were estimated as proportions. The time to DVT resolution was explored using survival analysis, as described before. Association between resolution and treatment modality was investigated using ordered logistic regression, a generalization of logistic regression for categorical dependent variables.21 To minimize the impact of confounding by indication, the model was adjusted for the number of vessels affected. Association between recurrent DVT and thrombophilia, stratified per group, was explored using the Fisher exact test and Cochran-Mantel-Haenszel statistics.
Significance level was set at P < .05.
Results
Two-hundred forty-four children were diagnosed with UE-DVT during the study period. Of these patients, 23 had a primary event and 221 had a secondary event. Within the secondary group, 86 patients had no clinic follow-up (5 neonates and 25 non-neonates died; 9 neonates and 47 non-neonates were transferred out). Hence our final cohort comprised 158 patients: 23 primary UE-DVT (G1), 25 neonatal secondary UE-DVT (G2neonates), and 110 non-neonatal secondary UE-DVT (G2non-neonates).
General characteristics of the cohort
The general characteristics of the cohort are listed in Table 1. The median follow-up time was significantly shorter in primary UE-DVT compared with the secondary groups. Patients in G1 were significantly older than patients in the secondary groups at the time of diagnosis of UE-DVT. The groups did not differ significantly in the male:female ratio. Patients in G1 were healthy at the time of diagnosis of UE-DVT. In contrast, surgical procedures and diagnosis of cancer occurred more frequently around the time of DVT in G2 patients. Half of the females in G1 (n = 6) were taking OCP at the time of UE-DVT diagnosis. Anthropometric measurements were not significantly different among the groups. Although no thrombophilia was documented in G2neonates, the overall frequency of patients in whom an abnormality was identified was 18% in G2non-neonates and 43% in G1.The majority of traits found were minor.
Characteristics of patients and of UE-DVT per group
| Variable . | G1 (n = 23) . | G2 neonates (n = 25) . | G2 non-neonates (n = 110) . | P value . |
|---|---|---|---|---|
| Age, median (25th, 75th percentile) | 16 y (15, 17 y) | 15 d (12, 23 d) | 3.7 y (3.2 mo, 10.7 y) | <.001* |
| Follow-up time, median (25th, 75th percentile) | 1.6 y (1.0, 2.1) | 3.7 y (2.0, 6.3) | 3.0 y (1.7, 4.9) | <.001* |
| Sex ratio (male:female) | 0.8 | 1.5 | 1.2 | .50† |
| BMI median (25th, 75th percentile) weight-for-length percentiles | 64 (42, 79) | −15 (<3-50) | 65 (26, 86) 25 (<3-50) | .99* |
| Underlying conditions | 0 | <.001‡ | ||
| None | 23 (100%) | 0 | 0 | |
| Postsurgical | 0 | 20 (80%) | 43 (38%) | |
| CHD | 0 | 15 (60%) | 23 (21%) | |
| Prematurity | 0 | 4 (16%) | 25 (23%) | |
| Cancer | 0 | 0 | 34 (31%) | |
| IBD | 0 | 0 | 10 (9%) | |
| Thrombophilia# | — | |||
| FVL homozygous | 1 (4%) | 0 | 1 (1%) | |
| FVL heterozygous | 5 (22%) | 0 | 5 (6%) | |
| PTG heterozygous | 1 (4%) | 0 | 3 (4%) | |
| AT deficiency | 0 | 0 | 1 (1%) | |
| PS deficiency | 0 | 0 | 1 (1%) | |
| APS | 1 (4%) | 0 | 2 (2%) | |
| Elevated factor VIII | 1 (4%) | 0 | 7 (8%) | |
| Elevated Lp(a) | 1 (4%) | 0 | 0 | |
| Causes of DVT | <.001‡ | |||
| Line-related | 0 | 25 (56% CVL, 44% PICC) | 101 (59% PICC, 22% CVL, 15% ports, 4% other) | |
| Effort/idiopathic | 23 (100%) | 0 | 0 | |
| Mass | 0 | 0 | 6 (5%) | |
| Other | 0 | 0 | 3 (3%) | |
| Symptoms of acute DVT | 23 (100%) | 3 (12%) | 55 (50%) | <.001† |
| Imaging modality for DVT | 5 (23%) | — | ||
| US | 24 (96%) | 95 (86%) | ||
| Venogram | — | 1 (4%) | 3 (3%) | |
| S+venogram | 5 (23%) | — | 7 (6%) | |
| US+MRV | 2 (9%) | — | — | |
| US+CT | — | — | 5 (5%) | |
| US+MRV+venogram | 11 (45%) | — | — | |
| Therapeutic modality for DVT | <.001† | |||
| Anticoagulant therapy | 15 (65%) | 25 (100%) | 92 (83%) | |
| Anticoagulant prophylaxis | 0 | 0 | 3 (3%) | |
| Thrombolysis | 8 (35%)§ | 0 | 2 (2%)¶ | |
| No treatment | 0 | 0 | 13 (12%) |
| Variable . | G1 (n = 23) . | G2 neonates (n = 25) . | G2 non-neonates (n = 110) . | P value . |
|---|---|---|---|---|
| Age, median (25th, 75th percentile) | 16 y (15, 17 y) | 15 d (12, 23 d) | 3.7 y (3.2 mo, 10.7 y) | <.001* |
| Follow-up time, median (25th, 75th percentile) | 1.6 y (1.0, 2.1) | 3.7 y (2.0, 6.3) | 3.0 y (1.7, 4.9) | <.001* |
| Sex ratio (male:female) | 0.8 | 1.5 | 1.2 | .50† |
| BMI median (25th, 75th percentile) weight-for-length percentiles | 64 (42, 79) | −15 (<3-50) | 65 (26, 86) 25 (<3-50) | .99* |
| Underlying conditions | 0 | <.001‡ | ||
| None | 23 (100%) | 0 | 0 | |
| Postsurgical | 0 | 20 (80%) | 43 (38%) | |
| CHD | 0 | 15 (60%) | 23 (21%) | |
| Prematurity | 0 | 4 (16%) | 25 (23%) | |
| Cancer | 0 | 0 | 34 (31%) | |
| IBD | 0 | 0 | 10 (9%) | |
| Thrombophilia# | — | |||
| FVL homozygous | 1 (4%) | 0 | 1 (1%) | |
| FVL heterozygous | 5 (22%) | 0 | 5 (6%) | |
| PTG heterozygous | 1 (4%) | 0 | 3 (4%) | |
| AT deficiency | 0 | 0 | 1 (1%) | |
| PS deficiency | 0 | 0 | 1 (1%) | |
| APS | 1 (4%) | 0 | 2 (2%) | |
| Elevated factor VIII | 1 (4%) | 0 | 7 (8%) | |
| Elevated Lp(a) | 1 (4%) | 0 | 0 | |
| Causes of DVT | <.001‡ | |||
| Line-related | 0 | 25 (56% CVL, 44% PICC) | 101 (59% PICC, 22% CVL, 15% ports, 4% other) | |
| Effort/idiopathic | 23 (100%) | 0 | 0 | |
| Mass | 0 | 0 | 6 (5%) | |
| Other | 0 | 0 | 3 (3%) | |
| Symptoms of acute DVT | 23 (100%) | 3 (12%) | 55 (50%) | <.001† |
| Imaging modality for DVT | 5 (23%) | — | ||
| US | 24 (96%) | 95 (86%) | ||
| Venogram | — | 1 (4%) | 3 (3%) | |
| S+venogram | 5 (23%) | — | 7 (6%) | |
| US+MRV | 2 (9%) | — | — | |
| US+CT | — | — | 5 (5%) | |
| US+MRV+venogram | 11 (45%) | — | — | |
| Therapeutic modality for DVT | <.001† | |||
| Anticoagulant therapy | 15 (65%) | 25 (100%) | 92 (83%) | |
| Anticoagulant prophylaxis | 0 | 0 | 3 (3%) | |
| Thrombolysis | 8 (35%)§ | 0 | 2 (2%)¶ | |
| No treatment | 0 | 0 | 13 (12%) |
APS, antiphospholipid syndrome; AT, antithrombin; CHD, congenital heart disease; CT, computed tomography; G1, primary UE-DVT group; G2, secondary UE-DVT group; IBD, inflammatory bowel disease; Lp(a), lipoprotein (a); MRV, magnetic resonance venogram; PICC, peripherally inserted central catheter; PS, protein S; US, Doppler ultrasound.
Mann-Whitney-Wilcoxon.
χ2.
Fisher exact test.
Local pharmaco-mechanical thrombolysis.
Systemic thrombolysis.
Patients tested for thrombophilia were n = 23 of 23 for G1, 16 of 25 for G2 neonates, and 84 of 110 for G2 non-neonates.
Characteristics of the UE-DVT at diagnosis
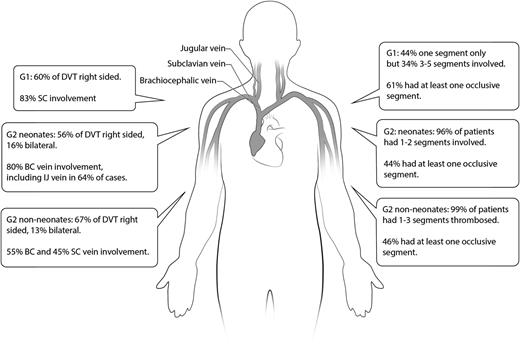
The majority of thrombotic events in G1 were effort-related (20/23; 87%) and potentially triggered by playing sports (swimming, golf, tennis, water polo, baseball, rowing), weight lifting, dancing, and instrument playing (drums, trumpet) (Table 1 and Figure 1). Conversely, most secondary UE-DVT cases were line-related (126/135; 93%). Thrombotic events were most commonly CVL-related in neonates and peripherally inserted central catheter (PICC)-associated in non-neonates. UE-DVT occurred predominantly on the right side, independently of the group. Bilateral events were more common in patients with congenital heart disease (CHD; 18/38, 56%) and cancer (5/35, 14%). The subclavian vein was the most common segment involved in G1, whereas brachiocephalic vein involvement was more frequent in G2. A larger number of vein segments were affected in G1. One-half to two-thirds of patients in all groups had at least one completely occluded segment at diagnosis.
Thrombosis distribution, laterality, and number of affected segments per group. BC, brachiocephalic vein; IJ, internal jugular vein; SC, subclavian vein.
Thrombosis distribution, laterality, and number of affected segments per group. BC, brachiocephalic vein; IJ, internal jugular vein; SC, subclavian vein.
Different from G2 patients, children on G1 had a combined imaging modality approach for DVT diagnosis. All G1 patients had symptoms at the time of the diagnosis of acute DVT. In contrast, only a minority of patients in G2neonates was symptomatic (3/25, 12%). Children in G2non-neonates were symptomatic in half of the cases (55/110, 50%). The most common sign in all groups was local edema.
Therapeutic modality
The majority of patients were treated with therapeutic anticoagulation (Table 1). Sixteen (15%) of patients in G2non-neonates were not treated or received prophylactic anticoagulation only, eight to treat asymptomatic or chronic thrombus, three because of the risk of bleeding, and five for unclear reasons. One-third (8/23) of patients in G1 received up-front local pharmaco-mechanical thrombolysis, followed by standard anticoagulation. Two of these 8 patients had minor bleeding events (at the sites of peripheral line insertion) during pharmaco-mechanical thrombolysis. Only 2 of 110 (2%) children in G2non-neonates received systemic thrombolysis. The overall median (25th and 75th percentiles) length of treatment was 6.7 months (5.0, 10.6 months) in G1, 90 days (85 days, 105 days) in G2neonates, and 94 days (87, 112 days) in G2non-neonates. The median (25th and 75th percentiles) time to start therapy was similar in all groups, at 0 days (0 and 1 day). Four G1 patients underwent surgical correction of thoracic outlet syndrome.
Outcomes
PTS.
Twenty patients (87%) in G1, 4 patients (16%) in G2neonates, and 54 (49%) patients in G2non-neonates had a PTS score ≥1 at the time of their last clinic follow-up (Table 2). The distribution of severities was significantly different among groups (P = .007).
Frequency of primary and secondary outcomes
| Outcome . | G1, n (%) . | G2 neonates, n (%) . | G2 non-neonates, n (%) . | P value . |
|---|---|---|---|---|
| PTS | .007 | |||
| No | 3 (13%) | 21 (84%) | 56 (51%) | |
| Mild | 14 (61%) | 4 (16%) | 52 (47%) | |
| Moderate | 6 (26%) | 0 | 2 (2%) | |
| Severe | 0 | 0 | 0 | |
| DVT resolution | .35 | |||
| Complete resolution | 7 (30%) | 10 (40%) | 38 (36%) | |
| Partial resolution | 13 (56%) | 9 (36%) | 37 (35%) | |
| No resolution or extension | 3 (13%) | 6 (24%) | 31 (29%) | |
| DVT recurrence | 3 (13%) | 0 | 15 (14%) | .12 |
| PE | — | |||
| Suspected | 4 (17%) | 0 | 8 (7%) | |
| Confirmed | 0 | 0 | 3 (2.7%) |
| Outcome . | G1, n (%) . | G2 neonates, n (%) . | G2 non-neonates, n (%) . | P value . |
|---|---|---|---|---|
| PTS | .007 | |||
| No | 3 (13%) | 21 (84%) | 56 (51%) | |
| Mild | 14 (61%) | 4 (16%) | 52 (47%) | |
| Moderate | 6 (26%) | 0 | 2 (2%) | |
| Severe | 0 | 0 | 0 | |
| DVT resolution | .35 | |||
| Complete resolution | 7 (30%) | 10 (40%) | 38 (36%) | |
| Partial resolution | 13 (56%) | 9 (36%) | 37 (35%) | |
| No resolution or extension | 3 (13%) | 6 (24%) | 31 (29%) | |
| DVT recurrence | 3 (13%) | 0 | 15 (14%) | .12 |
| PE | — | |||
| Suspected | 4 (17%) | 0 | 8 (7%) | |
| Confirmed | 0 | 0 | 3 (2.7%) |
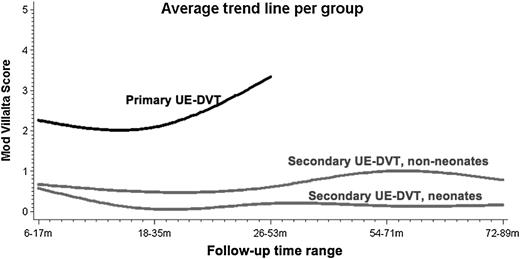
When analyzing the trajectory of PTS scores according to the longitudinal clinical assessment of each patient, it was found that PTS severity tended to increase in primary cases, whereas non-neonates showed only a mild severity increase. The average trend lines for PTS scores over follow-up time ranges are shown in Figure 2.
Increased limb circumference, pain, collaterals, and edema were the most common PTS-related clinical findings recorded in G1 patients, occurring in one-half to two-thirds of patients. Collateral vessels and increased limb circumference were the most common clinical findings in patients with secondary UE-DVT (36% and 16% in G2neonates and 46% and 27% in G2non-neonates, respectively).
Survival analysis showed that approximately half of the patients would be diagnosed with PTS one year (95% CI 0.7, 1.1), 9.7 years (95% CI 3.2, 11.0), and 3.1 years (95% CI 2.8, 3.6) after UE-DVT in G1, G2neonates, and G2non-neonates, respectively. The log-rank test showed a statistically significant difference in survival distribution among groups (P < .0001). Kaplan-Meier curves are shown in Figure 3.
Kaplan-Meier curves showing time (in years) to PTS according to group.
Simple logistic regression showed that the following predictors were significantly associated with PTS: group (G1, G2neonates, G2non-neonates, P < .001), age of the patient (P < .001), symptoms at DVT presentation (yes vs no, P < .001), subclavian vein involvement (yes vs no, P = .02), type of treatment (no treatment or prophylaxis, anticoagulation, thrombolysis, P = .003), and length of anticoagulation treatment (<45 days vs 45-90 days vs >90 days, P = .023). Conversely, sex (male vs female, P = .88), number of affected segments (P = .30), degree of vessel occlusion (occlusive vs nonocclusive, P = .45), Δt (in days, P = .61), resolution of DVT (complete or partial vs no resolution or extension, P = .09), recurrent DVT (yes vs no, P = .06), and the presence of thrombophilia (no thrombophilia vs major vs minor, P = .22) were not significantly associated with the outcome. No statistically significant association between DVT affecting the dominant arm and PTS was found (P = .66).
All potential predictors associated with the outcome at the prespecified P value of .20 were considered for a multivariable logistic regression model. According to the final model (Table 3), patients with primary UE-DVT were 37.7 times (95% CI, 10.7-133.6) as likely to develop PTS as patients with secondary UE-DVT of any age. Patients who had no resolution or extension of thrombosis at follow-up were 7.16 (95% CI, 1.5-33.4) times as likely as patients who had complete resolution to develop PTS.
Multivariable logistic regression model for the outcome PTS
| Predictor . | Adjusted OR (95% CI) . | P value . |
|---|---|---|
| Omnibus likelihood ratio | <.001 | |
| Primary UE-DVT vs secondary UE-DVT | 37.7 (10.7-133.6) | <.001 |
| Partial resolution vs complete resolution | 2.7 (0.6-11.0) | .17 |
| No resolution or extension vs complete resolution | 7.16 (1.5-33.4) | .01 |
| Predictor . | Adjusted OR (95% CI) . | P value . |
|---|---|---|
| Omnibus likelihood ratio | <.001 | |
| Primary UE-DVT vs secondary UE-DVT | 37.7 (10.7-133.6) | <.001 |
| Partial resolution vs complete resolution | 2.7 (0.6-11.0) | .17 |
| No resolution or extension vs complete resolution | 7.16 (1.5-33.4) | .01 |
OR, odds ratio.
DVT resolution.
Follow-up imaging showed partial resolution of the thrombotic event in the majority of G1 patients. Conversely, complete resolution was more frequent in the secondary groups (Table 2). Resolution status was not significantly different among groups (P = .35).
Ordered logistic regression showed that the degree of resolution at the end of therapy (complete vs partial resolution vs no resolution/extension), adjusted for the number of affected vein segments, was significantly associated with treatment modality (no treatment/prophylaxis only vs therapeutic anticoagulation vs thrombolysis, P = .012). The odds of no resolution/DVT extension were 6.5 times greater (95% CI 2.4-17.7, P < .001) than the odds of complete and partial resolution (combined) in patients who received no treatment/prophylaxis only, compared with patients who received full anticoagulation. The odds of no resolution/DVT extension in patients who received thrombolysis were not significantly different from complete and partial resolution combined, when compared with patients receiving therapeutic anticoagulation (OR 1.4; 95% CI, 0.04-5.0; P = .61). We found no association between the presence of thrombophilia and degree of resolution (P = .78).
Recurrent DVT.
The incidence of recurrent DVT is shown in Table 2. Patients had a recurrent event at a median (range) of 206 days (193-631) in G1 and 127 days (61-1716) in G2non-neonates. Two recurrent events in G1 and 3 events in G2non-neonates were incidentally found. There was no association between recurrence and thrombophilia (P = .4), even after stratifying for group (P = .20).
PE.
Twelve patients in the cohort (Table 2) had a ventilation-perfusion (V̇/Q̇) scan or an angio-pulmonary CT for suspected PE around the time of UE-DVT diagnosis. Three of these patients (G2non-neonates group) had an objectively confirmed PE, resulting in an incidence of 2.7% among non-neonates with secondary UE-DVT and of 1.9% in the entire cohort. These 3 patients had sustained line-related DVT; one had cancer, one had a metabolic disease, and the third patient was homozygous for FVL.
Deaths.
No DVT-related deaths were found in this cohort.
Discussion
Given the differences in the relative distribution of thrombotic events in upper and lower limbs between children and adults described before, UE-DVT is more relevant as a health care problem in the pediatric population. Our study describes the characteristics and outcomes of these events.
In agreement with our findings in G1 patients, the literature reports a predominance of subclavian vein compromise among adult patients who sustain UE-DVT.22-24 Regarding DVT laterality, studies have shown an increased frequency of left-side compromise in line-related UE-DVT in both adults and children, particularly in the setting of cancer.25-28 The increased frequency of right-sided events found herein at our institution is probably related to the higher frequency of line placement in the right arm.29
We found a high prevalence of thrombophilia traits in primary UE-DVT. Although still controversial, some studies suggest thrombophilia may play a role in UE-DVT in adults, especially in primary cases.30 Adult patients with inherited thrombophilia have been reported to have twice the odds of sustaining primary or secondary UE-DVT31 and 2 to 6 times the odds of sustaining primary UE-DVT32,33 compared with healthy controls. Interestingly, FVL and PTG have been reported to increase the odds of sustaining UE-DVT in adult OCP users.31,32 Our study design precludes analyzing such interaction.
DVT can be followed by long-term complications, including PTS.9 There are physiological differences between the UE and LE venous territories, such as in hydrostatic pressure and the number of vein valves,20,34,35 which likely affect not only the frequency but also the clinical features of PTS. To date, there is limited research focusing on the characteristics and predictors of UE-PTS.
To our knowledge, only 2 relatively small studies have focused specifically on pediatric UE-PTS. The 40% incidence of PTS among 15 children with primary UE-DVT reported by Malec10 is lower than the incidence found here in primary UE-DVT cases, which may be caused by limitations of the current instruments used to diagnose PTS.17 Conversely, the frequency of PTS among non-neonates with secondary UE-DVT observed here is similar to the 54% found by Kuhle et al in 13 patients with a history of acute leukemia.12
We found that the frequency of pediatric UE-PTS differed according to the pathogenesis of acute DVT and, within the secondary group, to the patient’s age. Teenagers with primary UE-DVT developed PTS more frequently and sooner than the secondary groups. In comparison, features compatible with PTS were least frequently observed in neonates with secondary UE-DVT and occurred almost a decade after the diagnosis of the thrombotic event. The low frequency of PTS among neonates may be associated with differences in the inflammatory response. Thrombosis and inflammation are closely related processes. For example, selectins, proteins that mediate the recruitment of neutrophils to inflammation sites,36 are also relevant in thrombotic events. Notably, P-selectin has a direct role in the early phases of thrombosis, and its inhibition has been shown to reduce perithrombotic inflammation and postthrombotic vein wall fibrosis in animal models, independent of the absolute size of the thrombus.37,38 Interestingly, neonatal neutrophils and neonatal endothelial cells have been shown to exhibit decreased P-selectin expression.39,40
Neonates also showed less severe PTS-related clinical findings compared with older children and adolescents in this study. Importantly, the impact of mild PTS is starting to be unraveled, with a recent study reporting that, unlike the more severe PTS cases, self-reported quality of life (QOL) of patients with mild PTS was similar to that of children without PTS, according to a generic QOL tool.41
Both the pathogenesis of acute DVT and the end-of-therapy DVT resolution predicted the development of PTS in our study. Resolution status was the only significant risk factor for UE-PTS found by Prandoni et al in a prospective cohort of 53 adults with primary or secondary UE-DVT.5
Thrombophilia, including persistent FVIII elevation, was not a predictor of PTS, nor was it associated with DVT recurrence or resolution as 3 independent outcomes in this cohort. Importantly, the results of available pediatric studies have not agreed on the role of elevated FVIII elevation as a risk factor for PTS.42-44
The frequency of DVT resolution observed here is similar to that previously reported among pediatric patients with non–central nervous system venous thrombosis45,46 and among children with arterial and venous thrombosis treated with standard anticoagulation.47
Thrombolysis did not result in a significant difference in DVT resolution in our study. Although research conducted in adult patients has shown more favorable thrombus resolution with the use of catheter-directed thrombolysis and systemic thrombolysis,48-52 the superiority of these modalities compared with standard anticoagulation in preventing UE-PTS is still unclear.1 Nonetheless, larger and specifically designed studies are required to investigate the impact of thrombolysis in pediatric UE-DVT.
Children with VTE are also susceptible to other severe complications including recurrent thrombosis, PE, and death.9,53 The 13% to 14% frequency of recurrent events found herein falls within the 5% to 18.5% recurrence range previously reported in unselected cases of pediatric VTE9,54-59 and is slightly higher than that reported in adult patients (2.3%-14.5%, depending on the length of follow-up and therapeutic management).32,60-63 In our study, thrombophilia was not associated with the risk of recurrent events. This is in keeping with the results of studies conducted in adult patients that reported no difference in the adjusted hazard ratio for UE-DVT recurrence in patients with and without thrombophilia.32,60
The overall incidence of PE in our cohort (1.9%) is lower than the overall 9.6% incidence of symptomatic PE reported among patients with thrombosis at another site by the Pediatric Canadian Registry.9 According to this registry, the subset of patients with line-related UE-DVT had a 15% incidence of PE,64 which is also higher than the 2.7% incidence of PE we found among non-neonates with secondary DVT. The difference could be explained in part by the detection techniques used.
Although the overall thrombosis-related mortality rate in pediatrics has been reported to be as high as 2.2%,9,54,55 we found no UE-DVT–related deaths in our cohort.
There are several limitations to our study. First, a retrospective design requires caution when collecting data. Thus, to increase accuracy, data were extracted by 3 data collectors and outcomes were assessed by 3 researchers. Second, there was a 13% non–VTE-related death rate and a 25% loss to follow-up in patients with secondary UE-DVT; it is possible that disease severity may have differed in these patients. Also, because only patients with PTS assessment were included, the selected population may comprise patients with more severe clinical findings. Nevertheless, our cohort reflects a real clinical scenario. Third, the presence of confounding by indication in the analysis of the effect of therapy on the outcomes, particularly in primary UE-DVT, cannot be ruled out, and patients with more extensive thrombotic burden may have been selected to undergo thrombolysis. We attempted to minimize this confounding effect by taking into account the number of affected segments as a surrogate of thrombosis severity. Fourth, although we used one of the 2 tools proposed by the ISTH (MVS), we were unable to apply the second instrument retrospectively (Manco-Johnson Instrument). Nonetheless, a recent study reported an almost perfect agreement between both tools (κ = 0.88).65
In conclusion, the results presented here highlight the importance of PTS as an emerging disease in pediatrics. Given the relative high frequency of UE-DVT, further investigation on the outcomes of such thrombotic events is required. Our study shows that secondary UE-DVT likely has a more benign course regarding the development of PTS compared with primary UE-DVT, particularly in neonates, and that residual thrombosis is also a risk factor for PTS development.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank Thomas Canil and Nisha Stephens for their help with data collection, and Jia Qu for designing Figure 1. Lucy Duan received the Young Investigator Award at the XXIV Congress of the ISTH, Amsterdam, Netherlands, 2013.
This study was supported by The University of Toronto and Sanofi-Aventis (L.D., A.C.); and a Baxter Bioscience Fellowship in Pediatric Hemostasis and Thrombosis at the Hospital for Sick Children, Toronto (M.L.A.).
Authorship
Contribution: M.L.A. collaborated with outcome assessment, performed data analyses, and wrote the manuscript; L.D., A.C., and A.K. collected the data; W.K. and S.W. critically reviewed the manuscript; and L.B. designed the study and wrote the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Leonardo R. Brandão, Division of Hematology/Oncology, The Hospital for Sick Children, Toronto, ON, Canada, M5G-1X8; e-mail: leonardo.brandao@sickkids.ca.