Key Points
Survival of patients with primary plasma cell leukemia has improved in recent years, but is still inferior to those patients with multiple myeloma.
This survival benefit appears to be mainly in patients older than 65 years of age.
Abstract
Primary plasma cell leukemia (pPCL) is a rare malignancy with an aggressive course and poor outcome. There has been significant improvement in the survival of multiple myeloma patients over the past decade as a result of incorporating autologous stem cell transplantation (ASCT) and novel agents into treatment regimens. However, it is unknown whether these therapies have had a similar impact on the survival of patients with pPCL. We conducted an analysis of the Surveillance, Epidemiology, and End Results database to evaluate the trends in survival of 445 patients with pPCL between 1973 and 2009. The widespread availability of ASCT and use of novel agents in the upfront setting of multiple myeloma and pPCL began after 1995 and 2006, respectively. The median overall survival based on periods of diagnosis were 5, 6, 4, and 12 months for those diagnosed during 1973-1995, 1996-2000, 2001-2005, and 2006-2009, respectively (P = .001). Thus, the current study confirms the recent survival improvement in pPCL within a large US population that may be associated with the use of better therapeutic strategies.
Introduction
Plasma cell leukemia (PCL) is a rare and aggressive malignancy defined by the presence of more than 20% circulating plasma cells and/or an absolute plasma cell count greater than 2 × 109/L in a peripheral blood smear.1-3 It accounts for approximately 1% to 2% of all plasma cell dyscrasias and presents in 2 forms, primary and secondary PCL. Primary PCL (pPCL) represents approximately 60% of PCL cases and presents de novo in the leukemic phase, without a prior diagnosis of multiple myeloma (MM). Secondary PCL (sPCL) accounts for the remaining 40% of PCL cases and indicates a “leukemic transformation” in approximately 1% of previously diagnosed and treated MM patients.4 Both forms of PCL have dismal outcomes, with the prognosis of patients with sPCL being even worse.5
The use of novel therapeutic agents such as immunomodulators (thalidomide and lenalidomide) and proteasome inhibitors (bortezomib),6 the incorporation of high-dose chemotherapy followed by autologous stem cell transplantation (ASCT) in eligible patients,7,8 and improved supportive care options have led to significant improvement in survival of MM patients.9 Unfortunately, improvement in survival has not been clearly demonstrated in pPCL as compared with MM. Prior descriptive studies on pPCL have suggested a median overall survival (OS) of less than 12 months based on patients who did not receive novel agents.1,10-13 However, recent retrospective studies suggest that the incorporation of novel agents as well as ASCT in treating pPCL has improved the median OS to more than 24 months.14-19 Given the lack of large, prospective studies evaluating the survival outcomes in pPCL because of its rarity, we used the Surveillance Epidemiology and End Results (SEER) program to evaluate the survival trends in pPCL patients in the US population during various time intervals based on the availability of ASCT and novel agent therapy.
Methods
SEER is a program of the National Cancer Institute that collects and publishes cancer incidence and survival data from 18 population-based cancer registries covering approximately 28% of the US population. Before 1992, there were 9 SEER reporting registries (SEER-9) including Atlanta, Connecticut, Detroit, Hawaii, Iowa, New Mexico, San Francisco-Oakland, Seattle-Puget Sound, and Utah. Since 1992, 9 more registries were added sequentially over time, including Los Angeles, San Jose-Monterey, Rural Georgia, the Alaska Native Tumor Registry, Greater California, Kentucky, Louisiana, New Jersey, and Greater Georgia to become the current SEER-18. The SEER public database 1973-2010 (SEER 18) based on the November 2012 submission (including July 2005-December 2005 Hurricane Katrina–impacted Louisiana cases) was used for this analysis (http://www.seer.cancer.gov). The SEER*Stat software (version 8.1.2; National Cancer Institute, Bethesda, MD) was used to obtain deidentified individual-level data from the SEER 18 registries via the case-listing survival session feature. All data were obtained and used in accordance with the SEER policies and procedures.
All PCL and MM cases were identified in the SEER cancer registry by International Classification of Diseases 0-3 codes 9733 (PCL) and 9732 (MM) for the 1973 to 2009 period. The year 2009 was selected as the last year of analysis for this study because the SEER cancer registry stopped coding for PCL separately from 2010 onward and have since been included in the same category as MM. Eligibility was restricted to patients who had microscopic confirmation of their disease, which was defined as verification of the diagnosis by tissue histology or cytology. Patients with no recorded age were not included. Those cases diagnosed at the time of death or through an autopsy were excluded. Similarly, patients with no follow-up or who were alive and had a survival time of 0 months were also excluded. In an attempt to assure that only pPCL cases were included in the analysis of PCL patients, each PCL case record was reviewed for other recorded malignancies and those that had a prior or concurrent diagnosis of MM (International Classification of Diseases 0-3 code: 9732) were excluded to eliminate any sPCL cases. OS was the primary end point of this study and was defined as the period from pPCL diagnosis to death from any cause and was determined from the SEER records of survival time (total number of months) and vital status (dead or alive).
The SEER database did not contain information on the types of therapies received by individual pPCL and MM patients. Therefore, patients were stratified based on the year of diagnosis into 4 cohorts (1973-1995, 1996-2000, 2001-2005, and 2006-2009) to study the impact of changing therapies. The period after 1995 represented adoption of ASCT as a standard treatment modality, and 2000 marked the start of novel agents such as bortezomib, lenalidomide, and thalidomide being available in the relapsed and refractory settings. Furthermore, it was not until 2006 that these novel agents were uniformly available in the first-line setting in MM.
Statistical analyses were performed using JMP 10.0.1 (SAS Institute Inc., Cary, NC). χ-square tests and Fisher exact tests were used to compare differences between nominal variables, and the Mann-Whitney U test or the Kruskal-Wallis test was used for continuous variables. Kaplan-Meier analysis was used to analyze and create the OS curves, and the log-rank test was used to compare survival curves. Finally, the Cox proportional hazards model was used to assess the influence of various prognostic factors on OS.
Results
Demographics of pPCL and MM patients
Among 6 576 644 malignant cases listed in the SEER 18 database between 1973 and 2009, there were 479 (<0.01%) patients with a diagnosis of PCL and 75 399 (1.1%) patients with a diagnosis of MM. After applying our exclusion criteria (as mentioned in “Methods”), the final analysis included only 445 PCL patients, all of whom were considered to have pPCL, and 66 862 MM patients. Demographic variables of these pPCL and MM patient cohorts are described and compared in Table 1. Among pPCL patients, the median age was 66 years (range, 19-98 years) and 54% were 65 years or older, both of which were lower than the MM patients diagnosed during the same period. Seventy-six percent of the pPCL patients were white and 51% were female. The demographic characteristics of the pPCL patients based on period of diagnosis is compared in Table 2.
Demographic characteristics of patients with pPCL and MM in the SEER 18 registry during the 1973-2009 period
| Variables (no., %) . | PCL (N = 445) . | MM (N = 66 862) . | P value . |
|---|---|---|---|
| Age (median, range) | 66 (19-98) | 69 (9-106) | <.001 |
| ≥65 y | 239 (54%) | 42 427 (63%) | |
| Male | 220 (49%) | 35 950 (54%) | .068 |
| Race | .096 | ||
| White | 339 (76%) | 51 306 (77%) | |
| Black | 90 (20%) | 11 738 (17%) | |
| Other | 16 (4%) | 3 818 (6%) | |
| Registry location | .135 | ||
| Northeast | 75 (17%) | 13 381 (20%) | |
| Midwest | 76 (17%) | 10 988 (16%) | |
| South | 80 (18%) | 9 691 (15%) | |
| West | 214 (48%) | 32 802 (49%) | |
| Period of diagnosis* | .002 | ||
| 1973-1995 | 120 (27%) | 22 171 (33%) | |
| 1996-2000 | 58 (13%) | 10 188 (15%) | |
| 2001-2005 | 131 (29%) | 18 396 (28%) | |
| 2006-2009 | 136 (31%) | 16 107 (24%) |
| Variables (no., %) . | PCL (N = 445) . | MM (N = 66 862) . | P value . |
|---|---|---|---|
| Age (median, range) | 66 (19-98) | 69 (9-106) | <.001 |
| ≥65 y | 239 (54%) | 42 427 (63%) | |
| Male | 220 (49%) | 35 950 (54%) | .068 |
| Race | .096 | ||
| White | 339 (76%) | 51 306 (77%) | |
| Black | 90 (20%) | 11 738 (17%) | |
| Other | 16 (4%) | 3 818 (6%) | |
| Registry location | .135 | ||
| Northeast | 75 (17%) | 13 381 (20%) | |
| Midwest | 76 (17%) | 10 988 (16%) | |
| South | 80 (18%) | 9 691 (15%) | |
| West | 214 (48%) | 32 802 (49%) | |
| Period of diagnosis* | .002 | ||
| 1973-1995 | 120 (27%) | 22 171 (33%) | |
| 1996-2000 | 58 (13%) | 10 188 (15%) | |
| 2001-2005 | 131 (29%) | 18 396 (28%) | |
| 2006-2009 | 136 (31%) | 16 107 (24%) |
P values in bold are statistically significant (P < .05).
Registry locations: West—Utah, New Mexico, Hawaii, California, Alaska, and Washington; Northeast—Connecticut and New Jersey; South—Louisiana, Kentucky, and Georgia; and Midwest—Iowa and Michigan.
Demographic characteristics of the 445 pPCL patients based on period of diagnosis
| Variables (no., %) . | All patients 1973-2009 (N = 445) . | Period of diagnosis . | ||||
|---|---|---|---|---|---|---|
| 1974-1995 (N = 120) . | 1996-2000 (N = 58) . | 2001-2005 (N = 131) . | 2006-2009 (N = 136) . | P value . | ||
| Age (median, range) | 66 (19-98) | 67 (19-93) | 66 (43-90) | 65 (21-98) | 66 (39-96) | .810 |
| ≥65 y | 239 (54%) | 66 (55%) | 32 (55%) | 67 (51%) | 74 (54%) | .918 |
| Male | 220 (49%) | 69 (58%) | 24 (41%) | 66 (50%) | 61 (45%) | .323 |
| Race | .120 | |||||
| White | 339 (76%) | 96 (80%) | 49 (84%) | 96 (73%) | 98 (72%) | |
| Black | 90 (20%) | 22 (18%) | 8 (14%) | 28 (22%) | 32 (24%) | |
| Other | 16 (4%) | 2 (2%) | 1 (2%) | 7 (5%) | 6 (4%) | |
| Registry location* | <.001 | |||||
| Northeast | 75 (17%) | 20 (17%) | 8 (14%) | 18 (14%) | 29 (21%) | |
| Midwest | 76 (17%) | 38 (32%) | 11 (19%) | 17 (13%) | 10 (7%) | |
| South | 80 (18%) | 10 (8%) | 7 (12%) | 25 (19%) | 38 (28%) | |
| West | 214 (48%) | 52 (43%) | 32 (55%) | 71 (54%) | 59 (44%) | |
| Variables (no., %) . | All patients 1973-2009 (N = 445) . | Period of diagnosis . | ||||
|---|---|---|---|---|---|---|
| 1974-1995 (N = 120) . | 1996-2000 (N = 58) . | 2001-2005 (N = 131) . | 2006-2009 (N = 136) . | P value . | ||
| Age (median, range) | 66 (19-98) | 67 (19-93) | 66 (43-90) | 65 (21-98) | 66 (39-96) | .810 |
| ≥65 y | 239 (54%) | 66 (55%) | 32 (55%) | 67 (51%) | 74 (54%) | .918 |
| Male | 220 (49%) | 69 (58%) | 24 (41%) | 66 (50%) | 61 (45%) | .323 |
| Race | .120 | |||||
| White | 339 (76%) | 96 (80%) | 49 (84%) | 96 (73%) | 98 (72%) | |
| Black | 90 (20%) | 22 (18%) | 8 (14%) | 28 (22%) | 32 (24%) | |
| Other | 16 (4%) | 2 (2%) | 1 (2%) | 7 (5%) | 6 (4%) | |
| Registry location* | <.001 | |||||
| Northeast | 75 (17%) | 20 (17%) | 8 (14%) | 18 (14%) | 29 (21%) | |
| Midwest | 76 (17%) | 38 (32%) | 11 (19%) | 17 (13%) | 10 (7%) | |
| South | 80 (18%) | 10 (8%) | 7 (12%) | 25 (19%) | 38 (28%) | |
| West | 214 (48%) | 52 (43%) | 32 (55%) | 71 (54%) | 59 (44%) | |
P values in bold are statistically significant (P < .05).
See the Table 1 footnote for registry locations.
Survival of pPCL patients
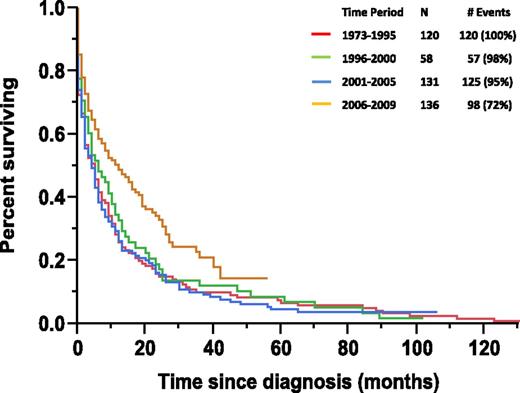
The median follow-up for the pPCL patient cohort was 85 months (range, 43-102) and 90% of patients had died at the time of this analysis. The median OS for the pPCL cohort was 6 months (95% confidence interval [CI]: 5-7), whereas the median 1-, 2-, and 5-year OS were 56%, 31%, and 5%, respectively. The median OS based on stratification by time periods of diagnosis including 1973-1995, 1996-2000, 2001-2005, and 2006-2009 were 5, 6, 4, and 12 months, respectively (P = .001) (Figure 1).
Kaplan-Meier Curve for OS in pPCL patients based on period of diagnosis.
Among the pPCL cohort, there were 101 (23%) patients who had a survival of less than 1 month, of which the majority was >65 years age (74 patients, P < .001) and male (62 patients, P = .006). The proportion of the pPCL patients dying within a month of their diagnosis during the 1973-1995, 1996-2000, 2001-2005, and 2006-2009 periods were 28%, 23%, 27%, and 15%, respectively (P = .043). In contrast, there were only 43 (10%) patients who had an OS of 3 or more years with equal distribution among the following periods (1973-1995: 10%, 1996-2000: 12%, 2001-2005: 10%, and 2006-2009: 8%; P = .852).
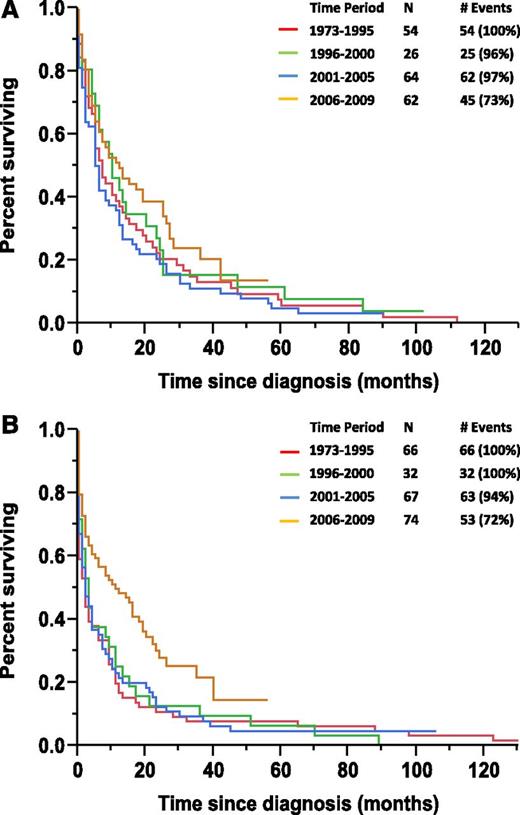
During the entire period of 1973-2009, the median OS was 7 months among patients <65 years compared with 3 months in patients >65 years (P = .026). However, during 2006-2010 alone, patients aged <65 years had a similar median OS of 12 months compared with 11 months in patients aged >65 years (P = .601). The median OS in patients <65 years age based on stratification by periods of diagnosis of 1973-1995, 1996-2000, 2001-2005, and 2006-2009 were 7 months, 10 months, 6 months, and 12 months, respectively (P = .193) (Figure 2A). Whereas the median OS in patients >65 years of age based on the similar stratification by periods of diagnosis of 1973-1995, 1996-2000, 2001-2005, and 2006-2009 were 2 months, 3 months, 2 months, and 11 months, respectively (P = .003) (Figure 2B).
Kaplan-Meier curves. (A) Kaplan-Meier curve for OS in pPCL patients based on period of diagnosis in patients younger than 65 years of age. (B) Kaplan-Meier curve for OS in pPCL patients based on period of diagnosis in patients 65 years of age or older.
Kaplan-Meier curves. (A) Kaplan-Meier curve for OS in pPCL patients based on period of diagnosis in patients younger than 65 years of age. (B) Kaplan-Meier curve for OS in pPCL patients based on period of diagnosis in patients 65 years of age or older.
OS prognostic variables in pPCL
Table 3 details the multivariable analysis of the 445 pPCL patients in which age <65 years, female gender, and diagnosis of pPCL during 2006-2009 predicted for an improved OS. However, race and location of diagnosis were not of prognostic significance for OS. Table 4 details the median OS of pPCL and MM patients based on age, gender, and period of diagnosis.
Prognostic variables affecting OS in patients with pPCL
| Variable . | OS . | |||
|---|---|---|---|---|
| Univariable analysis . | Multivariable analysis . | |||
| HR (95% CI) . | P value . | HR (95% CI) . | P value . | |
| Gender | .018 | .033 | ||
| Male | Reference | Reference | ||
| Female | 0.79 (0.65-0.96) | 0.80(0.66-0.98) | ||
| Age | .034 | .015 | ||
| <65 y | Reference | Reference | ||
| ≥65 y | 1.24(1.02-1.51) | 1.28(1.05-1.56) | ||
| Period of diagnosis | .002 | .002 | ||
| 1973-1995 | Reference | Reference | ||
| 1996-2000 | 0.93 (0.67-1.27) | 0.97 (0.70-1.33) | ||
| 2001-2005 | 1.03 (0.80-1.33) | 1.05 (0.82-1.36) | ||
| 2006-2009 | 0.65(0.49-0.85) | 0.66(0.50-0.87) | ||
| Race | .197 | — | — | |
| White | Reference | |||
| Black | 0.80 (0.62-1.03) | |||
| Other | 1.08 (0.61-1.74) | |||
| Registry location* | .861 | — | ||
| Northeast | Reference | |||
| Midwest | 1.14 (0.81-1.59) | |||
| South | 1.06 (0.76-1.48) | |||
| West | 1.11 (0.84-1.48) | |||
| Variable . | OS . | |||
|---|---|---|---|---|
| Univariable analysis . | Multivariable analysis . | |||
| HR (95% CI) . | P value . | HR (95% CI) . | P value . | |
| Gender | .018 | .033 | ||
| Male | Reference | Reference | ||
| Female | 0.79 (0.65-0.96) | 0.80(0.66-0.98) | ||
| Age | .034 | .015 | ||
| <65 y | Reference | Reference | ||
| ≥65 y | 1.24(1.02-1.51) | 1.28(1.05-1.56) | ||
| Period of diagnosis | .002 | .002 | ||
| 1973-1995 | Reference | Reference | ||
| 1996-2000 | 0.93 (0.67-1.27) | 0.97 (0.70-1.33) | ||
| 2001-2005 | 1.03 (0.80-1.33) | 1.05 (0.82-1.36) | ||
| 2006-2009 | 0.65(0.49-0.85) | 0.66(0.50-0.87) | ||
| Race | .197 | — | — | |
| White | Reference | |||
| Black | 0.80 (0.62-1.03) | |||
| Other | 1.08 (0.61-1.74) | |||
| Registry location* | .861 | — | ||
| Northeast | Reference | |||
| Midwest | 1.14 (0.81-1.59) | |||
| South | 1.06 (0.76-1.48) | |||
| West | 1.11 (0.84-1.48) | |||
P values in bold are statistically significant (P < .05). HR, hazard ratio.
See the Table 1 footnote for registry locations.
Survival of pPCL and MM patients based on gender, age, and period of diagnosis
| Age group . | Period . | Male . | Female . | ||
|---|---|---|---|---|---|
| Median OS . | P value . | Median OS . | P value . | ||
| <65 y | pPCL patients | ||||
| 1973-2005 | 5 mo | .219 | 11 mo | .179 | |
| 2006-2009 | 6 mo | 19 mo | |||
| MM patients | |||||
| 1973-2005 | 41 mo | <.001 | 44 mo | <.001 | |
| 2006-2009 | Not reached | Not reached | |||
| ≥65 y | pPCL patients | ||||
| 1973-2005 | 2 mo | .07 | 2 mo | .002 | |
| 2006-2009 | 9 mo | 12 mo | |||
| MM patients | |||||
| 1973-2005 | 20 mo | <.001 | 22 mo | <.001 | |
| 2006-2009 | 27 mo | 28 mo | |||
| Age group . | Period . | Male . | Female . | ||
|---|---|---|---|---|---|
| Median OS . | P value . | Median OS . | P value . | ||
| <65 y | pPCL patients | ||||
| 1973-2005 | 5 mo | .219 | 11 mo | .179 | |
| 2006-2009 | 6 mo | 19 mo | |||
| MM patients | |||||
| 1973-2005 | 41 mo | <.001 | 44 mo | <.001 | |
| 2006-2009 | Not reached | Not reached | |||
| ≥65 y | pPCL patients | ||||
| 1973-2005 | 2 mo | .07 | 2 mo | .002 | |
| 2006-2009 | 9 mo | 12 mo | |||
| MM patients | |||||
| 1973-2005 | 20 mo | <.001 | 22 mo | <.001 | |
| 2006-2009 | 27 mo | 28 mo | |||
P values in bold are statistically significant (P < .05).
Discussion
Our study used the most recent data available from the SEER program to evaluate survival trends in patients with pPCL among the US population. The median OS of patients with pPCL improved from 5 months when diagnosed before 2006 to 12 months when diagnosed between 2006 and 2009 (P < .001). Similarly, the early mortality (survival <1 month) rate dropped from 26% in pPCL patients diagnosed before 2006% to 15% when diagnosed after 2006 (P = .006). This is the first US population-based study to demonstrate a significant improvement in the OS of pPCL patients since 2006. However, the survival outcomes in pPCL patients are still inferior in comparison with MM patients diagnosed during the same period when adjusted for gender and age (Table 4).
Unlike previous studies in MM, our results do not demonstrate an improvement in survival of pPCL patients during 1996-2000 and 2001-2005 even though ASCT was approved as a treatment modality during these previously mentioned periods. This contradicts results by the Center for International Blood and Marrow Transplant Research, which demonstrated a 5-year OS of 51% in pPCL patients undergoing ASCT between 2000 and 2006. However, given the lack of treatment information such as ASCT utilization in the SEER registry, we are unable to know how many patients during 1996-2005 underwent ASCT and whether it was underused in the community during that period. It is possible that pPCL patients may have not been considered as candidates for ASCT because of persistent circulating plasma cells; however, the use of novel agents may have had a better “purging effect” than previous chemotherapy regimens, leading to an increased use of ASCT. Additionally, the retrospective Center for International Blood and Marrow Transplant Research analysis likely included a selection bias of those pPCL patients who were healthy enough to undergo an ASCT.
pPCL is a highly aggressive disease, with many of its patients presenting with severe complications leading to early death; ie, in 1 series, 11 of 40 patients died within the first month after diagnosis.20 Moreover, the survival of patients with pPCL has been historically short, with the median OS of patients treated without novel therapies ranging from 6 to 12 months.10-13 It is suggested that because of this aggressive course of pPCL, the need for treatment with a relatively early and sustained response is desirable.21 Recent single-institution studies suggest an improvement in OS among pPCL patients when novel agents have been incorporated upfront in their treatment.14-18 The wide availability of novel agents began in 2001; however, this was initially approved in the relapsed setting alone and it was not until 2006 that these agents began to gain approval for use in the first-line setting. Ramsingh et al assessed the outcomes of pPCL during 1973-2004 using the SEER 17 database but were unable to detect an improvement in OS with time.22 We hypothesize that because most of the novel agents were available for use in the upfront setting from 2006 onward, it was unlikely that many pPCL patients during 1973-2004 received upfront treatment with them. Whereas in our analysis of the SEER registry, we were able to detect an improvement in the OS of pPCL patients from 2006 onward compared with prior years. Unfortunately, the lack of treatment information in the SEER registry prevents confirmation of this hypothesis because the use of novel agents upfront vs at relapse cannot be clearly distinguished in our study. We were only able to use various time periods as a surrogate for potential novel agent use in the upfront and relapsed setting based on their utilization in the community.
Age >65 years was an expected and independent negative prognostic marker for OS in our study. However, our data also suggest that the OS benefit seen in pPCL patients diagnosed in the 2006-2009 period appears to have been derived mostly in patients >65 years (Figure 2A-B). This could be due to most of the mortality in older pPCL patients occurring within the first few months during the 1973-2005 period because they were either not candidates or had a poor tolerance to conventional chemotherapy; this is suggested by our data, which indicate that the majority of pPCL patients surviving less than 1 month were >65 years old. Furthermore, our results detect a decrease in the number of pPCL patients surviving less than 1 month from 2006-2009 onward in comparison with 1973-2005 (15% vs 26%). Together, these findings could suggest that the widespread use of novel agents in the upfront treatment of pPCL because 2006 led to an attenuation of the early mortality in these elderly patients. The lack of an overt improvement in OS in pPCL patients <65 years age diagnosed from 2006 to 2009 when compared with prior years may be partially explained by their relatively better median OS pre-2006 as well as their lower incidence of early mortality when compared with their elderly pPCL counterparts. Thus, there was less room for improvement in their median OS with the addition of novel agent therapy. Furthermore, given that the pPCL patients <65 years old already had access to ASCT in the years before the adaptation of upfront novel agent therapy, this provided them with better therapeutic options than the >65 years age pPCL patients who, before novel agent therapy, had no effective therapies.
In contrary to our suggestions of improved survival in pPCL because of the upfront use of novel agents, the analysis of 27 pPCL patients included in the Total Therapy trials (TT1, TT2, or TT3) by Usmani et al did not demonstrate a significant difference in OS between pPCL patients treated with or without novel agents. However, given that the majority of those pPCL patients (74%) was younger than 65 years, it could support our findings that most of the benefit in improving OS in pPCL has been experienced only by the elderly (>65 years). Furthermore, supportive care of patients with pPCL with regard to access to growth factor support and use of antimicrobial therapies for infectious complications since 2000 have remained unchanged and thus is unlikely to explain the improved survival seen in our study during the 2006-2009 period.
Despite the improvement in OS among pPCL patients, our study still shows that the 5-year survival rate from diagnosis continues to remain less than 10% and early mortality (survival <1 month) is 15% even if novel agents were available in the upfront setting, thus indicating the need for further research and development of newer therapeutic strategies and agents. This poor long-term survival rate is likely because pPCL is a much more aggressive disease with a higher tumor burden, plasma cell proliferative index, and incidence of extramedullary involvement than MM.21 Even though pPCL plasma cells share similar antigenic expression such as CD38 and CD138 with MM plasma cells, they are still distinct because they have a higher expression of CD20 and lower expression of CD9, CD117, CD56, and HLA-DR.11 The conventional cytogenetics of pPCL plasma cells are frequently complex and hypodiploidy with their fluorescence in situ hybridization analysis frequently demonstrating 1q+ and del17p abnormalities, all of which correlates with more aggressive and resistant disease.5 Similarly, gene expression profiling suggests differential expression of genes in pPCL plasma cells that result in a myeloid-like differentiation during leukemic development when compared with MM plasma cells.18
There are several limitations of this study, the first being that the SEER database does not contain information concerning the use of treatment types such as ASCT, chemotherapy, or novel agents; therefore, no direct assessment of a potential link between changes in therapy and superior survival results can be made. Because the use of novel agents upfront vs at relapse cannot be clearly distinguished in our study, we used various time periods as a surrogate for potential novel agent use in the upfront and relapsed setting based on the utilization of novel agents in the community. Nevertheless, the improvement in OS seen in the SEER data is similar to that reported in other smaller studies using novel agents in pPCL patients. Second, this remains a descriptive epidemiologic study of a population-based sample and not a randomized trial with predefined inclusion and exclusion criteria. Additionally, the registry includes a mix of patients who were treated at either community or academic facilities and thus likely causing heterogeneity in the treatment regimens administered. Third, the reliability and accuracy of the registry data are difficult to verify given the nature of this study especially in terms of the registry differentiating between PCL and MM and coding for them accurately. Finally, the registry lacks data on prognostic factors for pPCL to help determine other variables that could also impact OS.
In conclusion, though pPCL continues to be associated with worse long-term outcomes when compared with MM, it does appear that the survival of patients with pPCL has improved modestly since 2006. This could reflect the more frequent use of novel agents such as immunomodulators and proteasome inhibitors in the upfront management of the disease. However, given the lack of treatment information, we are unable to be certain of the survival benefit contributed by other modalities such as ASCT vs just using novel agents in the upfront setting alone. Nevertheless, future prospective multicenter clinical trials exploring newer agents and combination regimens will be necessary to improve the long-term survival in patients with this rare but fatal disease.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
This work was supported in part by the Mayo Clinic Hematological Malignancies Program; the National Cancer Institute (grants CA107476, CA62242, CA100707, and CA168762); the Jabbs Foundation; the Henry J. Predolin Foundation; and the National Center for Advancing Translational Sciences (Clinical and Translational Science Award, grant UL1 TR000135), a component of the National Institutes of Health.
The contents of this work are solely the responsibility of the authors and do not necessarily represent the official views of the National Institutes of Health.
Authorship
Contribution: S.K.K. and W.I.G. designed the study, collected and analyzed the data, and wrote the manuscript; and S.V.R., M.A.G., A.D., M.Q.L., P.P.S., V.G., F.K.B., R.S.G., D.D., S.R.H., J.A.L., S.R.Z., R.A.K., and P.K. contributed to writing and reviewing the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Shaji K. Kumar, Division of Hematology, Mayo Clinic, 200 First St SW, Rochester, MN 55905; e-mail: kumar.shaji@mayo.edu.