Key Points
Activation of platelet-TLR7 receptor mediates platelet-viral immune responses with no effect on thrombosis.
This is the first study to show that platelets are necessary for survival during viral infection.
Viral infections have been associated with reduced platelet counts, the biological significance of which has remained elusive. Here, we show that infection with encephalomyocarditis virus (EMCV) rapidly reduces platelet count, and this response is attributed to platelet Toll-like receptor 7 (TLR7). Platelet-TLR7 stimulation mediates formation of large platelet-neutrophil aggregates, both in mouse and human blood. Intriguingly, this process results in internalization of platelet CD41-fragments by neutrophils, as assessed biochemically and visualized by microscopy, with no influence on platelet prothrombotic properties. The mechanism includes TLR7-mediated platelet granule release, translocation of P-selectin to the cell surface, and a consequent increase in platelet-neutrophil adhesion. Viral infection of platelet-depleted mice also led to increased mortality. Transfusion of wild-type, TLR7-expressing platelets into TLR7-deficient mice caused a drop in platelet count and increased survival post EMCV infection. Thus, this study identifies a new link between platelets and their response to single-stranded RNA viruses that involves activation of TLR7. Finally, platelet-TLR7 stimulation is independent of thrombosis and has implications to the host immune response and survival.
Introduction
Platelets originate from megakaryocytes in the bone marrow and are released into the circulation as the second-most abundant blood component. Platelets are anucleated, highly granular cell fragments, prepackaged with proteins necessary for their function including small molecules such as serotonin, calcium, and adenosine 5′-diphosphate.1 The cytoplasm of platelets contains precursor-derived messenger RNAs (mRNAs), some of which are translated into proteins.2,3 Canonically, platelets have been associated with hemostasis or initiation of clot formation upon injury of the endothelium, thereby preventing leakage into the interstitial tissue. More recently, platelets have been connected to the host’s immune system via their ability to trap bacteria and present them to leukocytes for destruction.4
Systemic viral infections have long been associated with thrombocytopenia, and single-stranded RNA (ssRNA) viruses such as HIV,5 influenza,6 dengue,7 and hepatitis c virus,8 have been found inside human platelets. Clinical studies have shown that persistence of hepatitis c virus in patients’ platelets, post medical treatment, renders them prone to recurrent infection.8 Platelets are required for clearing infection with lymphocytic choriomeningitis virus and protecting the host against hemorrhagic diathesis.9 Dengue virus, in turn, induces platelet activation through stimulation of apoptotic caspase and mitochondrial dysfunction that explains the observed thrombocytopenia in patients.10 The medical significance of virally induced thrombocytopenia is unclear, as reduction in platelet count may be due to thrombosis or may lead to hemorrhage.
In humans, the Toll-like receptor (TLR) family contains 10 known members. TLR2 and TLR4 are surface receptors, whereas TLR3, TLR7, TLR8, and TLR9 are localized to the endosomal compartment of the cell.11 Endosomal TLRs are sensors for nucleic acid ligands: TLR3 is a sensor for double-stranded RNA, TLR9 is a sensor for DNA, and TLR7 is a sensor for ssRNA. Viruses such as encephalomyocarditis virus (EMCV), HIV, and influenza virus, as well as the small guanosine analogs loxoribine (Loxo) and imiquimod, activate the TLR7 receptor.11,12 Influenza virus-induced TLR7 activation requires the acidity of the endosomal compartment, which may facilitate exposure of the viral nucleotides for TLR7 detection. Platelets are known to express functional TLR2,13 TLR4,14 and TLR9,15,16 and activation of TLR2 or TLR4 leads to platelet-neutrophil interaction.13,14 Platelet-leukocyte communication has also been observed during different viral infections.17,,,,-22 Although platelets are known to express TLR2, TLR4, and TLR9, to date, there is no description of functional platelet-TLR7.
Given that ssRNA activates TLR7 and that infection with ssRNA viruses is associated with thrombocytopenia, we asked if platelet-TLR7 is actively involved in the decrease of platelet count. We evaluated the presence of TLR7 in platelets and whether its activation or elimination has a functional impact on viral immunity in vivo and/or on thrombotic-platelet responses. Here, we report that human and mouse platelets have a functional TLR7. Further, a 2-hour stimulation of this receptor induces an interaction of platelets with neutrophils and a reduction in platelet count. This process manifests as mild thrombocytopenia, but it is independent of pro-thrombotic behavior. Importantly, during viral infection, platelet presence is necessary to decrease mortality, and TLR7-exppressing platelets are sufficient to increase survival of mice that lack this receptor in all other tissues.
Methods
Identification of TLR7 in human platelets: The FHS analysis
A total of 1889 participants (839 men, 1050 women) of the Framingham Heart Study (FHS) Offspring Cohort (examination 8) examination were tested using quantitative polymerase chain reaction (qPCR) for the presence of TLR7 mRNA in their platelets (demographics are in supplemental Table 1, available on the Blood Web site). Purity of the platelet preparation was found to be <1 in 50 000 as previously described.23 Plasma P-selectin in these participants was correlated with mRNA levels as described in the supplemental Data. All participants signed informed consent, and the FHS protocol was approved by the Boston University Medical Center Institutional Review Board.23 This study was conducted in accordance with the Declaration of Helsinki.
Animal models and injections
All mice procedures were approved by University of Massachusetts Institutional Animal Care and Use Committee. TLR7 knockout (TLR7KO) mice were originally purchased from S. Akira24 and then backcrossed to C57BL/6J for at least 10 generations25 ; SELP (P-selectin) KO26 on C57BL/6J background and control mice were purchased from Jackson Laboratory (catalog nos. 002289 and 000664, respectively). This study used sex- and age-matched mice. The TLR7 agonist, Loxo (Invivogen), was dissolved in dimethyl sulfoxide, diluted in saline, and then injected at 2.5 μg/g of body weight. Saline control contained equivalent amount of dimethyl sulfoxide.
Platelet and neutrophil preparation
Platelets and neutrophils were isolated from freshly drawn venous blood or from blood drawn by heart puncture (in mice) as described in the supplemental Data. All procedures for human blood draw were approved by the University of Massachusetts Institutional Review Board.
Flow cytometry
Human heterotypic aggregation (HAG) was tested as follows: citrated human blood was diluted with phosphate-buffered saline in a 3:1 ratio, treated with different compounds for 15 minutes at room temperature in the presence of CD41–fluorescein isothiocyanate (FITC) (clone HIP8) and CD14–phycoerythrin cyanine 7 (PECy7) or CD14-PE (clone 61D3) or CD45-Cy5 (clone HI30) conjugated antibodies. HAGs in mice were tested in blood drawn 1 or 2 hours postinjection. Mouse blood was double-stained with CD41-FITC (clone eBioMWReg30) and Ly6G-PECy7-conjugated antibodies (clone RB6-8C5) at room temperature. All surface proteins discussed in this study were measured as described in the supplemental Data. Internalization of platelets was measured as described in the figures and analyzed by Amnis Flowsight Imaging Cytometer combined with a 20× confocal camera.
Microscopy
Collagen-adhesion slides were visualized using an inverted Olympus IX70 microscope. Slides with platelet-leukocyte aggregates were visualized by a spinning disk confocal Nikon TE2000E2 inverted microscope with motorized Z drive combined with Solamere Technology Groupmodified Yokogawa CSU10 Spinning Disk Confocal Scan Head with improved efficiency optics and Photometrics Coolsnap HQ2 camera with 1392 × 1040 pixel resolution. Detailed microscopy procedures done by scanning electron microscope (SEM) or transmission electron microscope (TEM) are described in the supplemental Data.
Platelet adhesion
Isolated washed platelets were resuspended in N-2-hydroxyethylpiperazine-N′-2-ethanesulfonic acid (HEPES) buffer (supplemental Data) and labeled with CalceinAM for 30 minutes as previously described.13 Labeled 2 × 108 platelets/500 mL were treated with different compounds for 15 minutes and recirculated over collagen-coated cover slips (500 μL of 100 μg/mL), for 20 minutes, using a cell-adhesion flow chamber (Immunetics, Boston, MA).
Platelet aggregation
Washed platelets (resuspended in HEPES buffer) or platelets from platelet-rich plasma at 2 × 108 platelets/500 mL were subjected to various concentrations of agonist treatments. Percent aggregation was measured by a PAP-4 Aggregometer from 8 to 20 minutes at 37°C as previously described.13
qPCR
Western-blot analysis
Protein from human or mouse platelets was isolated with RIPA buffer supplemented with protease and phosphatase inhibitors. Protein was processed as previously described27 and probed with primary antibodies as described in the supplemental Data.
Platelet depletion
Mice were injected with anti-platelet glycoprotein Ib beta chain (GPIb) and anti-immunoglobulin-G (IgG) antibodies (Emfret Analytics, Germany) at 2 ug of antibody/gram of mouse.28 Twelve hours postinjection mice were infected with EMCV.
CFSE platelet transfusion
Platelets were isolated from mice and labeled with 2 μg/mL carboxyfluorescein diacetate succinimidyl ester (CFSE) for 30 minutes at room temperature as previously described.29 Platelets were washed once with platelet wash buffer supplemented with prostaglandin E1 and then resuspended in HEPES and citrate-phosphate-dextrose (CPD) buffer in a 1:1 ratio (supplemented with prostaglandin E1). Platelets were injected in the tail vein of recipient mice at 108 platelets (in 100 μL) per 10 g of mice.29 At 2 hours posttransfusion, EMCV or Loxo (16 hours posttransfusion) were intraperitoneally injected.
EMCV murine infection
Male mice were infected with EMCV (1 × 107 plaque-forming units/200 μL of media). Control mice were injected with the media in which the virus was collected (Dulbecco’s modified Eagle medium, 10% heat-inactivated fetal calf serum, 1% l-Gln, 1% penicillin/streptomycin). Platelet number was measured at various times by collecting 15 μL of cheek blood diluted with 15 μL of CPD buffer.
Statistical analysis
All FHS data analysis was done using STATA. All other data were analyzed using GraphPad Prism 5. Details can be found in figure legends and supplemental Data.
Results
Platelets express functional TLR7, activation of which leads to reduction in platelet count
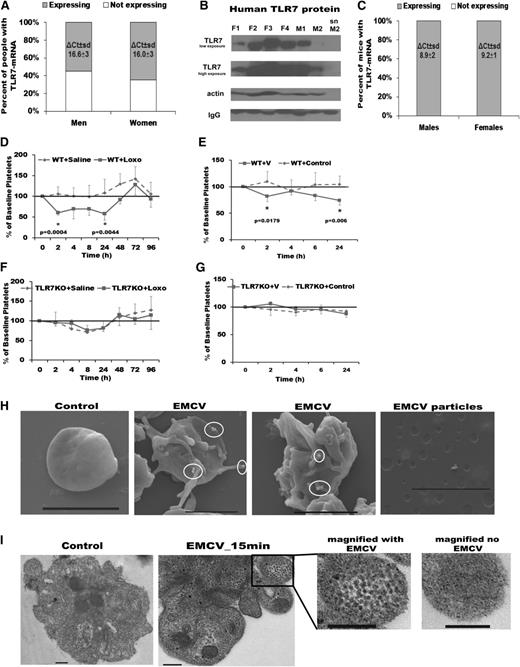
TLR7 detects and is activated by ssRNA viruses. Infections with ssRNA viruses in humans are associated with thrombocytopenia.30,31 To date, functional TLR7 in platelets has not been described. We analyzed platelet RNA derived from a large cohort associated with the FHS, including 839 men and 1050 women. An average of 60% (65% of women vs 55% of men) of the participants in this study had the TLR7-mRNA transcript in their platelets (Figure 1A). Western-blot analysis of platelets isolated from healthy donors (not involved in the FHS) showed presence of TLR7 protein in all samples, but levels were variable (Figure 1B). Exploring the reason for the variability of TLR7 expression observed in human platelets, while interesting, would be the focus of a different study. In contrast to the variable pattern in humans, all of the 11 mice tested had detectable levels of TLR7-mRNA in their platelets (Figure 1C).
TLR7 is present in human and mouse platelets and TLR7 activation induces a reduction in platelet count. (A) TLR7 mRNA levels in human platelets from participants in the FHS (n = 1889, 839 men and 1050 women) screened by qPCR. (B) TLR7 protein levels in human platelets of 6 healthy donors (not involved in the FHS) screened by western-blot analysis (F, females; M, men). (C) TLR7 mRNA levels in murine platelets screened by qPCR (n = 5 males, n = 6 females). (D-G) WT (baseline platelet count 729 ± 167 × 103 platelets/μL) and TLR7KO male mice (baseline platelet count 780 ± 147 × 103 platelets/μL) at 14 weeks of age were injected (once) with TLR7 agonist, Loxo or at 17 weeks of age with EMCV. Percent of baseline platelet count in WT (D) and TLR7KO (E) mice injected with Loxo and WT (F) and TLR7KO (G) mice infected with EMCV. SEMs (H) and TEMs (I) of human platelets treated with EMCV. White circles in H encompass viral particles. Bar represents 2 μm in H and 0.2 μm in I. Data are average ± SD and were analyzed by Student t test using n = 5 animals/group (C-H).
TLR7 is present in human and mouse platelets and TLR7 activation induces a reduction in platelet count. (A) TLR7 mRNA levels in human platelets from participants in the FHS (n = 1889, 839 men and 1050 women) screened by qPCR. (B) TLR7 protein levels in human platelets of 6 healthy donors (not involved in the FHS) screened by western-blot analysis (F, females; M, men). (C) TLR7 mRNA levels in murine platelets screened by qPCR (n = 5 males, n = 6 females). (D-G) WT (baseline platelet count 729 ± 167 × 103 platelets/μL) and TLR7KO male mice (baseline platelet count 780 ± 147 × 103 platelets/μL) at 14 weeks of age were injected (once) with TLR7 agonist, Loxo or at 17 weeks of age with EMCV. Percent of baseline platelet count in WT (D) and TLR7KO (E) mice injected with Loxo and WT (F) and TLR7KO (G) mice infected with EMCV. SEMs (H) and TEMs (I) of human platelets treated with EMCV. White circles in H encompass viral particles. Bar represents 2 μm in H and 0.2 μm in I. Data are average ± SD and were analyzed by Student t test using n = 5 animals/group (C-H).
To test if specific activation of the TLR7 receptor causes reduction in platelet count in vivo, we injected mice with a TLR7-agonist (Loxo) and monitored platelet counts in animals for 8 days. Compared with saline-injected control mice, there was a sudden 45% drop in platelet count at 2 hours postinjection and at 24 hours in wild-type (WT) mice (Figure 1D). Similarly, infection with EMCV led to a reduction in platelet count at 2 and 24 hours postinjection (Figure 1E). In both cases, the observed effect on platelet count was TLR7 specific, as Loxo or EMCV injection in TLR7KO mice did not affect platelet numbers compared with saline-injected TLR7KO mice (Figure 1F-G). Cell-surface sialic acid is a major entry receptor for this virus,32 and platelets are extensively coated with it.33 SEMs and TEMs revealed EMCV particles on the surface and inside of platelets with activated morphology (Figure 1H-I; supplemental Figure 1A-B). The presence of functional platelet-TLR7, internalization of viral particles by platelets, and a rapid drop in platelet count post TLR7 activation suggest an active involvement of platelet-TLR7 in viral response and in the subsequent platelet reduction.
Stimulation of platelet-TLR7 in mice leads to increased interaction of platelets with granulocytes
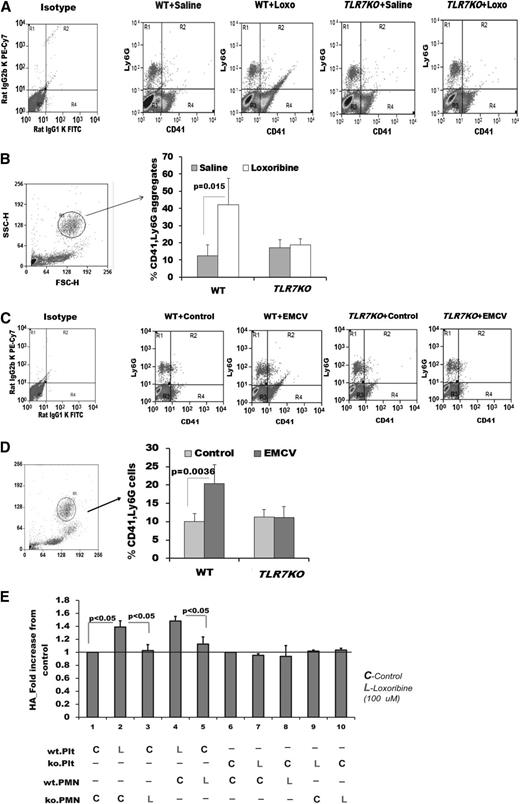
Platelet-TLR2 and TLR4 are known to stimulate formation of platelet-leukocyte aggregates, termed HAGs.13,34 To determine the mechanism by which platelets are rapidly reduced in the circulation during TLR7 stimulation, we examined whether TLR7 activation may increase platelet interaction with leukocytes. Platelet-leukocyte aggregation in murine blood was screened by flow cytometry at 1 hour after TLR7 agonist (Loxo) injection. At this time point, there was an increase in the percent of platelets associated with the granulocyte (Figure 2A-B) and monocyte and lymphocyte populations (supplemental Tables 2 and 3). An increase in the percent of platelets interacting with the granulocyte population was also observed at 2 hours post-EMCV infection in WT mice. There was no significant increase in the interaction with the monocyte or lymphocyte populations (Figure 2C; supplemental Tables 4 and 5). At 4 hours postinfection, platelet-leukocyte HAGs were no longer detected (Figure 2C; supplemental Figure 2). Platelet-granulocyte interaction was TLR7 specific, as challenge with Loxo or EMCV did not increase HAG formation in the blood of TLR7KO mice. Because (1) neutrophils compose ∼40% to 60% of the total white blood cell (WBC) population in mice, (2) the highest increase in interaction between platelets and leukocytes is with granulocytes (98% neutrophils) and, (3) platelet-neutrophil aggregates might significantly contribute to the initial drop in platelet count, we tested whether platelets have a role in the formation of platelet-neutrophil HAGs. To this end, a series of mixing experiments were performed using platelets and granulocytes from WT and TLR7KO mice isolated by Ficoll gradient. Only platelet-TLR7 was able to initiate the formation of aggregates with the granulocytes, and neutrophil-TLR7 did not have an effect on HAG formation (Figure 2E). This suggests that when TLR7 is activated (with Loxo or EMCV), platelets are the blood component that initiates communication with the neutrophil population.
Murine platelet-TLR7 mediates aggregation of platelets with leukocytes. Male mice were injected with Loxo. One hour postinjection, blood was drawn by cheek puncture, and aggregates between platelets (labeled with CD41) and leukocytes (labeled with Ly6G) were measured by flow cytometry. (A) Dot plots of each condition used with no gating. (B) Quantitation of CD41, Ly6G aggregates, gated around the granulocyte population. (C-D) Male mice were injected with EMCV as detailed in “Methods.” (C) Dot plots of each condition used with no gating. (D) Quantitation of CD41, Ly6G-positive aggregates, gated around the granulocyte population. Data are average ± SD and were analyzed by Student t test using n = 6 (3 males, 3 females)/group/condition (B) and n = 4 (D). (E) Isolated platelets or neutrophils (abbreviated as PMN) were labeled, pretreated, and then mixed for 15 minutes with the other untreated population. The Ly6G, CD41-positive population was quantified by flow cytometry n = 3 (2 mice per isolation were pooled for n = 1 in either genotype). KO denotes TLR7KO in E. Data are average ± SD and were analyzed by 1-way ANOVA (P < .0001) followed by Bonferroni test.
Murine platelet-TLR7 mediates aggregation of platelets with leukocytes. Male mice were injected with Loxo. One hour postinjection, blood was drawn by cheek puncture, and aggregates between platelets (labeled with CD41) and leukocytes (labeled with Ly6G) were measured by flow cytometry. (A) Dot plots of each condition used with no gating. (B) Quantitation of CD41, Ly6G aggregates, gated around the granulocyte population. (C-D) Male mice were injected with EMCV as detailed in “Methods.” (C) Dot plots of each condition used with no gating. (D) Quantitation of CD41, Ly6G-positive aggregates, gated around the granulocyte population. Data are average ± SD and were analyzed by Student t test using n = 6 (3 males, 3 females)/group/condition (B) and n = 4 (D). (E) Isolated platelets or neutrophils (abbreviated as PMN) were labeled, pretreated, and then mixed for 15 minutes with the other untreated population. The Ly6G, CD41-positive population was quantified by flow cytometry n = 3 (2 mice per isolation were pooled for n = 1 in either genotype). KO denotes TLR7KO in E. Data are average ± SD and were analyzed by 1-way ANOVA (P < .0001) followed by Bonferroni test.
Activation of human platelet-TLR7 leads to platelet-granulocyte HAG formation and requires acidic pH found in the intracellular vesicles
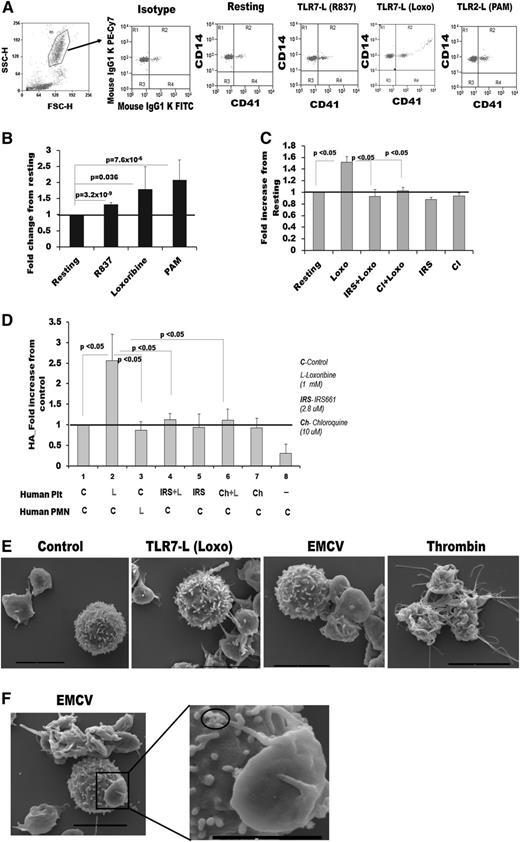
To examine if human TLR7 is able to initiate formation of HAGs, similarly to what we found with mouse platelets, we collected venous blood from donors and stimulated with TLR7 agonists (Loxo and R837). The percent of CD41-labeled platelets found associated with leukocytes significantly increased after TLR7 stimulation, and platelets were found with both the granulocyte and monocyte populations (Figure 3A-B; supplemental Figure 3A). In this experiment, we used the TLR2 agonist, Pam3CSK4, as a positive control.13 To further test if the HAG formation in human blood is TLR7 specific, TLR7 was inhibited with IRS661.35 This inhibition prevented platelets from adhering to the granulocyte population (Figure 3C) but was inefficient in inhibiting the interaction with monocytes (data not shown). TLR7 is an endosomal receptor and requires the acidic pH of the endosome to bind viral RNA. Platelets, however, are not known to have endosomes but contain primary lysosomes that harbor an acidic pH.1,36 To test if TLR7 is located in acidic vesicles and mediates HAG formation by signaling from intracellular compartments, we used chloroquine. Cloroquine is a compound that accumulates in the endosome-like vesicles and raises the pH to be more basic, thereby inhibiting any signaling that originates from these vesicle compartments. Pretreatment with chloroquine abolished HAG formation (Figure 3C), suggesting that TLR7 signals originate from intracellular acidic vesicles. To test if human platelet-TLR7 is also responsible for the initiation of HAG formation with neutrophils, platelets and granulocytes were isolated, separately pretreated, and then mixed together. Only activation of platelet-TLR7 was able to initiate the interaction with granulocytes in an endosomal-like fashion (Figure 3D). Activation of granulocyte-TLR7 was not sufficient to induce HAGs, which is consistent with previous studies showing that human neutrophils are poorly activated by Loxo.37 SEM imaging of incubated platelets and WBCs confirmed HAG formation in the presence of Loxo or EMCV (Figure 3E-F; supplemental Figure 3B-C). Importantly, platelets activated by TLR7 (presumably performing immune function) appeared with extended pseudopodia and ruffles. These TLR7-induced changes were markedly different than the morphological changes observed in thrombin-treated platelets (platelets performing their hemostatic function). These data suggest that in humans, similarly as in mice, platelet-TLR7 also mediates the interaction of platelets with granulocytes and that platelets are actively involved in the initial response to EMCV viral infection.
Human platelet-TLR7 mediates aggregation of platelets with granulocytes through endosomal signaling. Blood from human donors was treated with Loxo immediately after draw and stained with CD41 (platelets) and CD14 (leukocytes) antibodies. Aggregates between platelets and granulocytes were measured by flow cytometry. (A) Dot plots gated around the granulocyte population. FSC-H, forward scatter; SSC-H, side scatter. (B) Quantitation of CD41, CD14-positive aggregates from A. The following conditions were applied: R837 (2 μg/mL; n = 8); Loxo (1 mM; n = 5); Pam3CSK4 (10 μg/mL; males n = 10). (C) CD41, CD14 aggregates in human blood (n = 3) posttreatment with specific antagonist (IRS661, 2.8 μM) or the endosomal inhibitor chloroquine (Cl, 10 μM). (D-E) Platelets (denoted Plt) and neutrophils (denoted PMN) were isolated from human blood and labeled with CD41 and CD14, respectively. (D) Quantitation of CD41, CD14-positive aggregates in each fraction stimulated with either TLR7 agonist (Loxo) or pretreated with IRS661 or Cl (for 30 minutes) and then stimulated with Loxo for 15 minutes. The fractions were mixed together for 15 minutes, and aggregates were measured (n = 4) in each experiment. (E) SEM images of platelets and WBC stimulated together with different agonists. (F) SEM image of a WBC and a platelet with viral particles at the end of its pseudopodia (see circles). Bar in E and F represents 4 μm, except in the magnified image, where it is 2 μm. Data are average ± SD and were analyzed by Student t test (3B) or 1-way ANOVA (C, P < .05; E, P < .0001) followed by Bonferroni test (P values are noted on the graph).
Human platelet-TLR7 mediates aggregation of platelets with granulocytes through endosomal signaling. Blood from human donors was treated with Loxo immediately after draw and stained with CD41 (platelets) and CD14 (leukocytes) antibodies. Aggregates between platelets and granulocytes were measured by flow cytometry. (A) Dot plots gated around the granulocyte population. FSC-H, forward scatter; SSC-H, side scatter. (B) Quantitation of CD41, CD14-positive aggregates from A. The following conditions were applied: R837 (2 μg/mL; n = 8); Loxo (1 mM; n = 5); Pam3CSK4 (10 μg/mL; males n = 10). (C) CD41, CD14 aggregates in human blood (n = 3) posttreatment with specific antagonist (IRS661, 2.8 μM) or the endosomal inhibitor chloroquine (Cl, 10 μM). (D-E) Platelets (denoted Plt) and neutrophils (denoted PMN) were isolated from human blood and labeled with CD41 and CD14, respectively. (D) Quantitation of CD41, CD14-positive aggregates in each fraction stimulated with either TLR7 agonist (Loxo) or pretreated with IRS661 or Cl (for 30 minutes) and then stimulated with Loxo for 15 minutes. The fractions were mixed together for 15 minutes, and aggregates were measured (n = 4) in each experiment. (E) SEM images of platelets and WBC stimulated together with different agonists. (F) SEM image of a WBC and a platelet with viral particles at the end of its pseudopodia (see circles). Bar in E and F represents 4 μm, except in the magnified image, where it is 2 μm. Data are average ± SD and were analyzed by Student t test (3B) or 1-way ANOVA (C, P < .05; E, P < .0001) followed by Bonferroni test (P values are noted on the graph).
Platelet-TLR7 initiates p38-MAPK and Akt phosphorylation leading to α-granule release
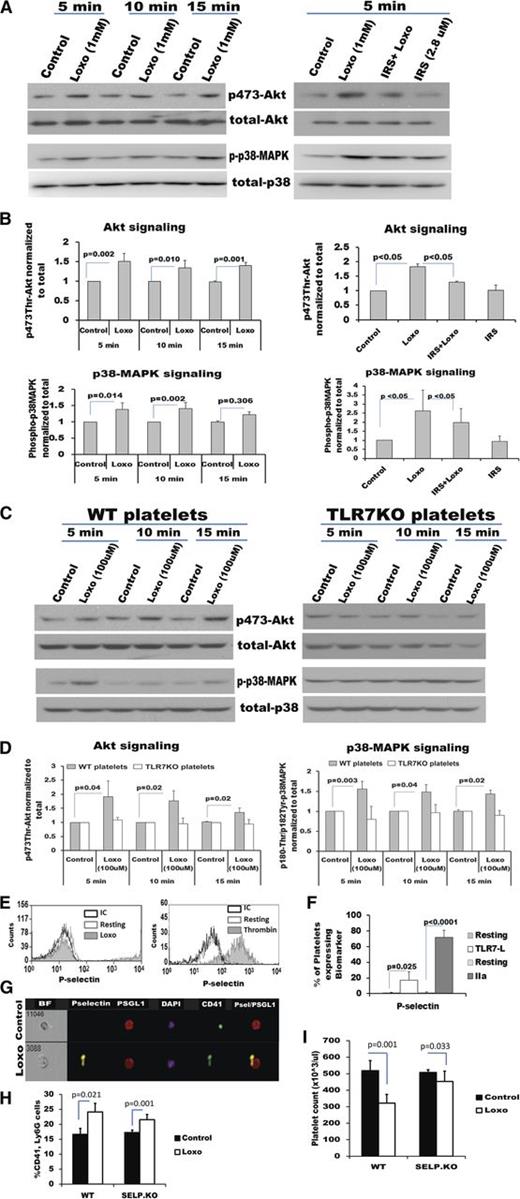
To determine the functional consequence of TLR7 activation, in terms of HAG formation, the platelet intracellular cell signaling mechanism was evaluated. Platelets lack a nucleus, and kinases such as p38 mitogen-activated protein kinase (MAPK) and Akt do not initiate nuclear events, but cause α-granule release.38,-40 This process is described as platelet activation and is manifested by translocation of granule proteins (ie, P-selectin) to the surface, thereby leading to interaction with other cells. Western-blot analysis of TLR7-stimulated human or murine platelets showed that p38MAPK and Akt are phosphorylated as early as 5 minutes posttreatment with Loxo (Figure 4A-C). Furthermore, the effect on phosphorylation was decreased by antagonism of human TLR7 or abolished in the TLR7KO mice (Figure 4A-D). Following TLR7 agonist activation, there was a significant increase in surface expression of P-selectin and CD40L, proteins responsible for the interaction of platelets with WBCs1,41 (Figure 4E-F; supplemental Figure 4A-B). Platelets expressing surface P-selectin post-TLR7 stimulation interacted with P-selectin glycoprotein ligand-1 on the granulocyte population (Figure 4G). However, not all platelets interacting with WBCs were P-selectin positive (data not shown). Consistently, P-selectin KO mice had a reduced ability to interact with the neutrophil population after TLR7 stimulation (Figure 4H). The reduction in platelet count in the P-selectin KO mice at 2 hours post TLR7-agonis injection was only 11% (WT mice ∼42%) (Figure 4I). Supportive of the experimental findings in mice, human platelet-TLR7 mRNA expression was positively correlated with plasma P-selectin levels in the participants of the FHS (Table 1). We conclude that TLR7 is functional in platelets; it initiates phosporylation on intracellular kinases and consequently translocation of ligands responsible for interaction with WBCs (P-selectin and CD40L).
TLR7 activation in platelets leads to Akt and p38-MAPK phosphorylation and translocation of P-selectin to the cell surface. (A-D) Platelets were isolated from humans and mice and treated with TLR7 agonist (Loxo) or pretreated (for 30 min) with the TRL7 antagonist, IRS661, and then stimulated with Loxo for the indicated time intervals. Protein was isolated and resolved by western-blot analysis. Phosphorylation (A) and quantitation (B) of kinases involved in α-granule release in human platelets. (C-D) Phosphorylation and quantitation, respectively, of kinases involved in α-granule release in murine platelets. (E) P-selectin surface expression and (F) quantitation in human platelets, post TLR7 agonist (Loxo) or thrombin (IIA) stimulation, resolved by flow cytometry. IC, isotype control. (G) Platelet P-selectin interacts with PSGL-1 on the granulocyte population measured by FlowSight image cytometer. (H-I) Five-week-old female WT and P-selectin (SELP) KO mice were injected with Loxo. (H) Heterotypic aggregates between CD41-platelets and Ly6G-PMNs and platelet count (I) 2 hours after Loxo injection. Data are average ± SD and were analyzed by Student t test, except the quantitation of the experiment with the inhibitors, analyzed by ANOVA (P < .0001). Analysis is based on n = 4 humans (A-B), n = 3 groups of mice (C-D), n = 4 for Loxo and n = 3 for thrombin (E), n = 4 (F), and n = 3 (G), n = 3-4 mice/group (H-I).
TLR7 activation in platelets leads to Akt and p38-MAPK phosphorylation and translocation of P-selectin to the cell surface. (A-D) Platelets were isolated from humans and mice and treated with TLR7 agonist (Loxo) or pretreated (for 30 min) with the TRL7 antagonist, IRS661, and then stimulated with Loxo for the indicated time intervals. Protein was isolated and resolved by western-blot analysis. Phosphorylation (A) and quantitation (B) of kinases involved in α-granule release in human platelets. (C-D) Phosphorylation and quantitation, respectively, of kinases involved in α-granule release in murine platelets. (E) P-selectin surface expression and (F) quantitation in human platelets, post TLR7 agonist (Loxo) or thrombin (IIA) stimulation, resolved by flow cytometry. IC, isotype control. (G) Platelet P-selectin interacts with PSGL-1 on the granulocyte population measured by FlowSight image cytometer. (H-I) Five-week-old female WT and P-selectin (SELP) KO mice were injected with Loxo. (H) Heterotypic aggregates between CD41-platelets and Ly6G-PMNs and platelet count (I) 2 hours after Loxo injection. Data are average ± SD and were analyzed by Student t test, except the quantitation of the experiment with the inhibitors, analyzed by ANOVA (P < .0001). Analysis is based on n = 4 humans (A-B), n = 3 groups of mice (C-D), n = 4 for Loxo and n = 3 for thrombin (E), n = 4 (F), and n = 3 (G), n = 3-4 mice/group (H-I).
Association between plasma P-selectin levels and mRNA expression of genes in human platelets from the FHS (cohort 8), n = 1889
| Platelets . | P-selectin in plasma (beta ± SD, P value) . |
|---|---|
| MYD88 | 0.002 ± 0.002, P = .417 |
| TLR2 | −0.001 ± 0.002, P = .661 |
| TLR4 | −0.005 ± 0.003, P = .117 |
| TLR7 | 0.007 ± 0.002, P = .004 |
| TLR8 | 0.004 ± 0.002, P = .064 |
| Platelets . | P-selectin in plasma (beta ± SD, P value) . |
|---|---|
| MYD88 | 0.002 ± 0.002, P = .417 |
| TLR2 | −0.001 ± 0.002, P = .661 |
| TLR4 | −0.005 ± 0.003, P = .117 |
| TLR7 | 0.007 ± 0.002, P = .004 |
| TLR8 | 0.004 ± 0.002, P = .064 |
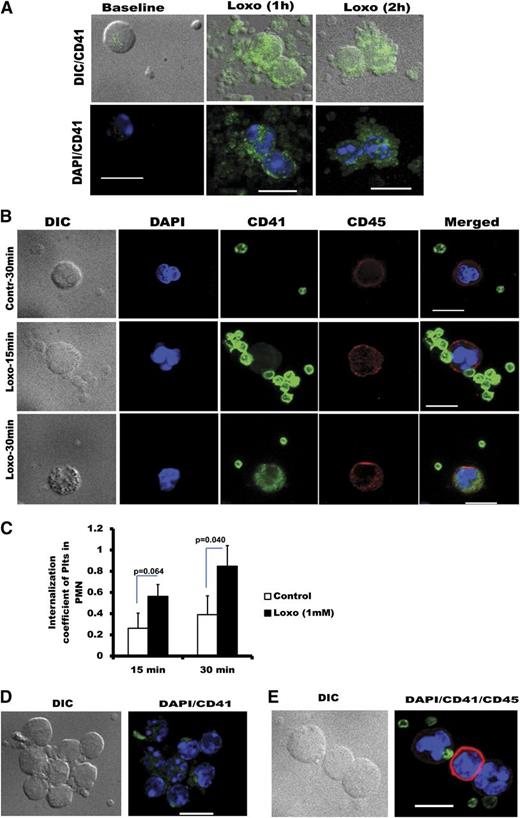
Rapid reduction in platelet count, caused by TLR7 stimulation, leads to internalization of platelet-CD41 by granulocytes and large aggregate formation with other leukocytes
Neutrophils have been described to incorporate platelet microparticles42 and engulf activated platelets in vivo.43 Interestingly, in mice, there is no change in WBC count at 2 hours postinjection with either Loxo or EMCV (supplemental Figure 5A-D). This suggests that, at this early time point, platelets are more likely to still be in the circulation in the form of uncleared HAGs. To visualize the presence of TLR7-stimulated HAGs in the circulation (at 2 hours postinjection), platelets from WT mice were fluorescently labeled with CFSE,29 transfused into WT mice, and then mice were injected with Loxo. One hour post-Loxo injection, CFSE-labeled platelets formed large aggregates with the neutrophil population (identified by the lobularity of the neutrophil nucleus) (Figure 5A). Two hours postinjection, platelets were still associated with the neutrophils, but CFSE was completely internalized within the neutrophils, suggesting internalization of platelet fragments in vivo (Figure 5A). To assess if this phenomenon of platelet internalization occurs in human blood, human platelets and WBCs were prelabeled (CD41 and CD45, respectively) immediately after phlebotomy, and then blood was stimulated with Loxo for 15 and 30 minutes. Confocal microscopy demonstrated that platelets form large aggregates with neutrophils at 15 minutes, and platelet-CD41 can be found within the neutrophils at 30 minutes poststimulation. (Figure 5B; supplemental Figure 5E-G; supplemental Video 1). To quantify the internalization platelet-CD41 in neutrophils (identified as CD14.dim, CD3−, DAPI.high), an internalization coefficient was determined by the IDEAS software of Amnis imaging cytometer. When compared with control, treatment of human blood with Loxo increased the internalization coefficient of CD41 in the granulocyte population from 0.23 to 0.56 at 15 minutes and from 0.28 to 0.73 at 30 minutes (Figure 5C; supplemental Figure 5H). Loxo stimulation of human blood and Loxo injection in mice transfused with CFSE platelets showed that platelets are involved in the formation of large aggregates with various WBCs (Figure 5D-E). Our results indicate that the observed reduction in platelet count 2 hours post TLR7 activation is most likely due to platelet interaction with the leukocyte population and internalization of platelet fragments by neutrophils.
Rapid thrombocytopenia induced by TLR7 stimulation is caused by granulocyte (neutrophil) internalization of platelets and continued leukocyte aggregate formation. (A) Confocal microscopy of murine blood postinjection with Loxo. Mice were transfused with CFSE-labeled platelets; blood was drawn, fixed, and stained with 4,6 diamidino-2-phenylindole (DAPI). (B) Confocal microscopy of human blood prestained with CD41-FITC and CD45-CY5 (CD41-green stains are platelets and CD45-red stains are leukocytes) and stimulated with Loxo. DAPI (blue) stains the nucleus and was added postfixation. Cells are identified as neutrophils according to their lobularity and intensity of CD45. (C) Internalization coefficient measured by IDEAS-software (Amnis FlowSight Cytometry) in human blood. In these studies, CD45 was used to define the outside of the neutrophils, and CD41 is the internalization probe (n = 4). (D-E) Confocal images of human or murine blood showing large aggregates of platelets and WBCs. (D) Murine blood of mice transfused with CFSE-labeled platelets and injected with Loxo, fixed, and stained with DAPI. (E) Human blood treated ex vivo with Loxo (15 minutes) and stained with CD41 (green)-platelets, CD45 (red)-leukocytes, or DAPI (blue)-nucleus. In all cases, pictures are representative of: n = 3 (A), n = 5 (B); n = 4 (C), n = 3 (D), and n = 5 (E). Data in C are average ± SD and were analyzed by Student t test. Images in A-B and D-E were taken with spinning disk confocal microscope and Metamorph 7.4.2 software and merged by ImageJ (NIH) software. Pictures were taken with ×100 Plan Apo oil lens and the scale bar is 10 μm.
Rapid thrombocytopenia induced by TLR7 stimulation is caused by granulocyte (neutrophil) internalization of platelets and continued leukocyte aggregate formation. (A) Confocal microscopy of murine blood postinjection with Loxo. Mice were transfused with CFSE-labeled platelets; blood was drawn, fixed, and stained with 4,6 diamidino-2-phenylindole (DAPI). (B) Confocal microscopy of human blood prestained with CD41-FITC and CD45-CY5 (CD41-green stains are platelets and CD45-red stains are leukocytes) and stimulated with Loxo. DAPI (blue) stains the nucleus and was added postfixation. Cells are identified as neutrophils according to their lobularity and intensity of CD45. (C) Internalization coefficient measured by IDEAS-software (Amnis FlowSight Cytometry) in human blood. In these studies, CD45 was used to define the outside of the neutrophils, and CD41 is the internalization probe (n = 4). (D-E) Confocal images of human or murine blood showing large aggregates of platelets and WBCs. (D) Murine blood of mice transfused with CFSE-labeled platelets and injected with Loxo, fixed, and stained with DAPI. (E) Human blood treated ex vivo with Loxo (15 minutes) and stained with CD41 (green)-platelets, CD45 (red)-leukocytes, or DAPI (blue)-nucleus. In all cases, pictures are representative of: n = 3 (A), n = 5 (B); n = 4 (C), n = 3 (D), and n = 5 (E). Data in C are average ± SD and were analyzed by Student t test. Images in A-B and D-E were taken with spinning disk confocal microscope and Metamorph 7.4.2 software and merged by ImageJ (NIH) software. Pictures were taken with ×100 Plan Apo oil lens and the scale bar is 10 μm.
Platelet-TLR7 alters survival in virally infected mice
To assess if platelets are actively involved in survival postviral infection, we used a model in which mouse platelets are depleted with an antibody against GPIb-α.28,44 Mice that had depleted platelets (supplemental Figure 6A) at the time of infection had increased mortality compared with their IgG-injected controls (Figure 6A). Of note, the antiplatelet antibody did not affect mortality in noninfected mice (Figure 6A) or WBC count at time of infection (supplemental Figure 6B). To evaluate the role of platelet-TLR7 on survival, in vivo heterotypic aggregate formation, and the reduction in platelet count, we performed a series of platelet transfusion experiments. TLR7-expressing platelets from WT mice were isolated, labeled with CFSE, and transfused into TLR7KO mice. After transfusion, mice were infected with EMCV and survival was monitored. The presence of TLR7-expressing platelets in the TLR7KO mice, even at low levels (∼10% of the endogenous platelets), significantly prolonged the survival time of the infected TLR7KO mice (Figure 6B). Platelets lacking the receptor, in turn, did not alter the survival of the infected WT mice (Figure 6C), as the endogenous TLR7 is still present and can induce a response. The presence of WT platelets in the TLR7KO mice was able to induce a moderate reduction in platelet count; however, TLR7KO platelets did not affect the platelet counts in WT mice (Figure 6D-E). Finally, after viral stimulation, the presence of platelet-TLR7 led to an increase in HAG formation with the neutrophil population in KO mice, an increase that had not been previously observed in the TLR7KO mice with viral infection (Figure 6C,F). These results strongly suggest that presence of TLR7 in platelets is necessary for initiating the innate immune response toward ssRNA viral infections, and the reduction of platelet count at early stages of viral infections involves platelet-TLR7.
Platelets and platelet-TLR7 mediate infection-affected mortality. WT male mice (17 weeks) were depleted of platelets by injecting anti-GPIb-α antibody (2 μg/g of mouse), and at 12 hours postinjection they were infected with EMCV. (A) Survival of EMCV-infected, platelet-depleted mice (n = 13) compared with IgG-injected and infected controls (n = 12) evaluated by Mantel-Cox test. Noninfected anti-IgG (n = 4) or anti-Plt (n = 4) injected mice had a 100% survival. (B-F) Platelets from WT or TLR7KO mice (abbreviated KO here) were isolated, labeled with CFSE, and transfused in recipient mice (all mice had similar CFSE-labeled platelets posttransfusion assessed by flow cytometry) as labeled on the graphs. The control mice were transfused with buffer in which platelets were concentrated. Mice were injected with EMCV 2 hours posttransfusion. (B-C) Survival curves of the injected mice determined by Mantel-Cox test and based on: n = 5 for WT->KO and n = 6 for buffer->KO, and n = 6 for KO->WT and n = 5 for buffer->WT. (D) Reduction in platelet count in the transfused KO (see Figure 1F) mice as a result of platelet-TLR7 presence. (E) Platelet count in the WT mice as a result of KO platelet transfusion. (F) Heterotypic aggregates induced in the WT and the KO mice post buffer transfusion. Data for D-F are average ± SD and were analyzed by 2-tail Student t test, based on the following n: (D-E) n = 4 (WTplt->KO), n = 5 (buffer->KO), n = 5 (KOplt->WT), and n = 3 (buffer->WT) (F) WT groups: n = 3 (control), n = 3 (EMCV), n = 3 (control), and n = 5 (EMCV).
Platelets and platelet-TLR7 mediate infection-affected mortality. WT male mice (17 weeks) were depleted of platelets by injecting anti-GPIb-α antibody (2 μg/g of mouse), and at 12 hours postinjection they were infected with EMCV. (A) Survival of EMCV-infected, platelet-depleted mice (n = 13) compared with IgG-injected and infected controls (n = 12) evaluated by Mantel-Cox test. Noninfected anti-IgG (n = 4) or anti-Plt (n = 4) injected mice had a 100% survival. (B-F) Platelets from WT or TLR7KO mice (abbreviated KO here) were isolated, labeled with CFSE, and transfused in recipient mice (all mice had similar CFSE-labeled platelets posttransfusion assessed by flow cytometry) as labeled on the graphs. The control mice were transfused with buffer in which platelets were concentrated. Mice were injected with EMCV 2 hours posttransfusion. (B-C) Survival curves of the injected mice determined by Mantel-Cox test and based on: n = 5 for WT->KO and n = 6 for buffer->KO, and n = 6 for KO->WT and n = 5 for buffer->WT. (D) Reduction in platelet count in the transfused KO (see Figure 1F) mice as a result of platelet-TLR7 presence. (E) Platelet count in the WT mice as a result of KO platelet transfusion. (F) Heterotypic aggregates induced in the WT and the KO mice post buffer transfusion. Data for D-F are average ± SD and were analyzed by 2-tail Student t test, based on the following n: (D-E) n = 4 (WTplt->KO), n = 5 (buffer->KO), n = 5 (KOplt->WT), and n = 3 (buffer->WT) (F) WT groups: n = 3 (control), n = 3 (EMCV), n = 3 (control), and n = 5 (EMCV).
Stimulation of platelet-TLR7 increases adhesion to collagen but does not promote thrombotic behavior
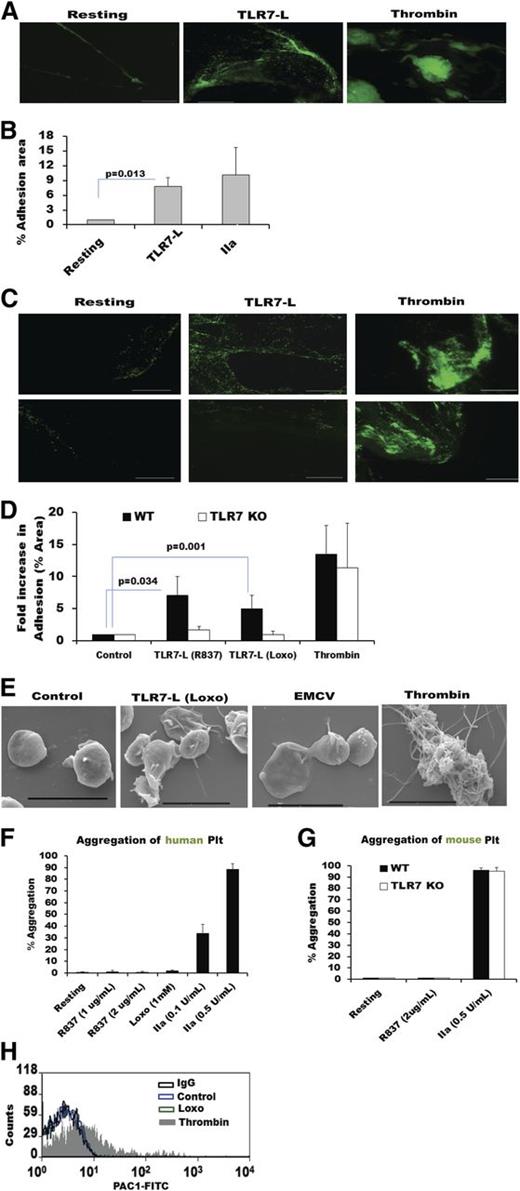
Upon injury to the endothelium, collagen is exposed, and platelets become activated as they attach to the vessel wall. The subsequent formation of a thrombus is due to platelets interacting with each other in a 3-dimensional manner. This normal hemostatic function of platelets can be pathological if the thrombus obstructs normal blood flow. To test if TLR7 affects the initial stage of hemostasis, platelets from human donors, or mice, were isolated, and their ability to adhere to collagen was evaluated. Activation of TLR7 by specific ligands (Loxo or R837) increased platelet adherence to collagen but to a lesser extent than when platelets were simulated with thrombin (Figure 7A-B). Platelets lacking TLR7 were not able to increase their attachment to collagen compared with control, and activation with thrombin had a similar effect on TLR7-deficient and WT platelets (Figure 7C-D). In support of this observation, SEMs of human platelets showed that thrombin induces tight clot formation, whereas Loxo or EMCV promoted a more loose type of interaction among platelets (Figure 7E). To test if TLR7 has an effect on the prothrombotic platelet function, platelet-platelet interactions were measured by aggregation. Neither washed platelets nor platelets in platelet-rich plasma (from human or mouse origin) aggregated in response to TLR7 ligands (Figure 7F-G). Further, pretreatment with TLR7 ligand did not affect the ability of platelets to aggregate in the presence of a low thrombin concentration (0.005 U/mL) or collagen (data not shown). In addition, we were not able to observe an increase in binding of PAC-1 after stimulation of platelet-TLR7 (Figure 7H). PAC-1 is an antibody used to test platelet aggregation/activation. The antibody recognizes an epitope on the αIIbβ3 integrin, which becomes exposed after platelet activation, and is necessary for fibrinogen binding. Finally, a bleeding test indicated that TLR7KO mice have prolonged bleeding times (232 ± 80 seconds, n = 3) compared with WT controls (102 ± 29 seconds, n = 4). This manifestation of overbleeding is also evident in Figure 1E, where the saline-injected TLR7KO controls exhibit a slight reduction in platelet count. Together, these experiments indicate that, although the initial adhesion to collagen can be affected as a result of activation of TLR7, direct pro-thrombotic events do not appear to be platelet-TLR7 dependent. This is in distinction to stimulation of the other platelet-TLRs, TLR213 /TLR445 /TLR9,15,16 predominantly activated by bacteria.
TLR7-activated platelets adhere to collagen but do not aggregate or form significant thrombi. Fluorescently labeled and stimulated, washed platelets were run over collagen-coated slides. (A) Human platelets treated with TLR7 ligand (TLR7-L) (R837, 2 μg/mL or 8.3 μM) or with thrombin (0.5 U/mL). (B) Quantitation of (A) for n = 6 individuals (3 females and 3 males). (C) Murine platelets treated with thrombin (0.5 U/mL) or TLR7-L: Loxo (100 μM) or R837 (2 μg/mL). (D) Quantitation of (C) using n = 3 (Loxo), n = 5 (R837), and n = 5 (thrombin). (E) SEM images of platelets treated with different agonists. (F) Aggregation of isolated human or (G) murine platelets treated with various concentrations of agonists depicted in the graph. Data are average ± SD and were analyzed by Student t test using n = 5 for human platelets (except Loxo, n = 3) and n = 3 for each condition in mice, as each n is pooled platelets from 4 to 5 mice. (H) PAC-1 binding to isolated human platelets post isotype control, control, Loxo (1 mM), and thrombin (0.005 U/mL) tested by flow cytometry. Histogram is representative of n = 4 individuals. PAC-1 tests for epitope on αIIbβ3 integrin of human platelets, necessary for fibrinogen binding and aggregation. Bars in A and D represent 100 μm and in E represent 4 μm.
TLR7-activated platelets adhere to collagen but do not aggregate or form significant thrombi. Fluorescently labeled and stimulated, washed platelets were run over collagen-coated slides. (A) Human platelets treated with TLR7 ligand (TLR7-L) (R837, 2 μg/mL or 8.3 μM) or with thrombin (0.5 U/mL). (B) Quantitation of (A) for n = 6 individuals (3 females and 3 males). (C) Murine platelets treated with thrombin (0.5 U/mL) or TLR7-L: Loxo (100 μM) or R837 (2 μg/mL). (D) Quantitation of (C) using n = 3 (Loxo), n = 5 (R837), and n = 5 (thrombin). (E) SEM images of platelets treated with different agonists. (F) Aggregation of isolated human or (G) murine platelets treated with various concentrations of agonists depicted in the graph. Data are average ± SD and were analyzed by Student t test using n = 5 for human platelets (except Loxo, n = 3) and n = 3 for each condition in mice, as each n is pooled platelets from 4 to 5 mice. (H) PAC-1 binding to isolated human platelets post isotype control, control, Loxo (1 mM), and thrombin (0.005 U/mL) tested by flow cytometry. Histogram is representative of n = 4 individuals. PAC-1 tests for epitope on αIIbβ3 integrin of human platelets, necessary for fibrinogen binding and aggregation. Bars in A and D represent 100 μm and in E represent 4 μm.
Discussion
It is well accepted that platelets are crucial for hemostasis. Bacterial and viral infections have been associated with varying intensity of thrombocytopenia due to differences in host response and/or infectious agents. Interestingly, platelets are able to trap bacteria leading to bacterial destruction by activation of TLR4 and TLR2.13,34,46 It has been proposed that viral thrombocytopenia in humans is due to the destruction of platelets by the immune system or by the suppression of platelet production.19 Here, we show that TLR7 activation in mice, by a specific agonist or EMCV infection, can initiate a reduction in platelet count as early as 2 hours posttreatment or postinfection. The cause of this early onset of viral platelet count reduction is TLR7 mediated, and it is most likely due to platelet aggregate formation with leukocytes, followed by internalization in the neutrophil population. Interestingly, we found platelet presence to be instrumental during viral infection, resulting in increased survival. Additionally, during EMCV infection, platelet-TLR7 induces interaction with the granulocytes and is able to alter the mortality rate of mice lacking the receptor. Neutrophil activation has been predominantly associated with a response to bacterial invasion, thus the finding that TLR7-stimulated platelets primarily interact with neutrophils was unexpected. Until this study, it was not known that platelets are the initiating agents of communication with the neutrophils during viral infection. Platelets are also the second-most abundant blood component and most likely are the first to encounter and sequester viral particles, making platelets a primary contributor to the innate immune response.
The immune role of platelets, however, is not without consequence. Thrombocytopenia occurs with viral infections in both humans and mice, and there is concern that viral infections may contribute to hemorrhage.47 Here, we show that a reduction in platelet count is a direct outcome of platelet-TLR7 stimulation, and it has a beneficial effect on survival. It is notable that there is a measurable difference between the extent to which platelet count is reduced when mice are injected with a TLR7 agonist or EMCV. This is not surprising, because viruses also activate other pathogen recognition receptors such as retinoic acid-inducible gene I-like receptors and nucleotide oligomerization domain-like receptors.48 Further, the decrease in platelet count at 24 hours may be due to a transient effect on platelets both directly via platelet-TLR7 and indirectly via other host receptor-dependent signals.49 Consistently, with the drop in platelet count at 24 hours, there is a concomitant increase in WBC number that is not observed in the TLR7KO mice. This increase in WBC number is probably due to the immune system responding to the active infection. Lastly, the difference in platelet count at 7 days post-TLR7 stimulation (168 hours) could be due to an inhibitory effect of TLR7 on thrombopoiesis or platelet release from the megakaryocytes.
Platelets were previously known to express functional TLR7. In a large observational cohort, we show that human platelets express the TLR7 mRNA transcript, and platelets from all healthy donors respond to the activation of platelet-TLR7, as evident by the functional adhesion data, mixing experiments, and platelet surface protein expression. The observed mismatch between TLR7 mRNA, TLR7 protein, and functional TLR7 responses suggests that most of the TLR7 protein is prepackaged by the megakaryocytes prior to platelet release.
It is important to note that platelet aggregation is not affected by direct stimulation of platelet-TLR7. Because platelet-platelet interactions during aggregation are crucial for the progression of thrombus formation, we postulate that platelet-TLR7 activation lacks prothrombotic properties. It will be of great interest, however, to evaluate if downstream effects of the environment during viral infections or TLR7 simulation can alter the progression of thrombus formation.
An important question raised by our study is whether the use of TLR7 agonists for the treatment of viral infections would lead to relevant reductions in platelet count or enhance the host immune response. Indeed, TLR7 activation is necessary to reduce viral load, produce protective host antibodies, and reduce severity of infection from ssRNA viruses such as with Ross River virus50 or lymphocytic choriomeningitis virus.51 We are the first to demonstrate that platelets are crucial for improving the survival of the host. Finally, we propose that platelets, due to their abundance, may be the first blood component to encounter viral particles and initiate the beginning of the innate immune response, particularly involving the neutrophil population. Future work is necessary to elucidate whether TLR7 activation has a direct effect on platelet production and/or platelet clearance. This study shows a novel role for platelets in mediating a response to ssRNA viruses without engagement of their pro-thrombotic function. This particular observation is imperative in the development of pharmaceutical drugs that aim to improve survival postinfection with TLR7-activating viruses.
The online version of this article contains a data supplement.
There is an Inside Blood Commentary on this article in this issue.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
We thank the University of Massachusetts Medical School Flow Cytometry, Digital Light Microscopy, and Electron Microscopy Cores for their training and assistance.
This work was supported by the National Institutes of Health, National Heart, Lung, and Blood Institute (HL-112311-02 to J.E.F., HL087201 to J.E.F., RFA-HL-12-008 to J.E.F., HL80442 to K.R., N01-HC 25195 to E.J.B., 1R01AG028321 to E.J.B.), National Institute of Allergy and Infectious Diseases (P01 AI078894 to J.E.F. and L.M.B., AI083215 to E.A.K.-J.), and National Heart, Lung, and Blood Institute T32 grant HL007224 to M.K.
Authorship
Contribution: M.K. designed and performed the experiments, interpreted the results, and wrote the manuscript; O.V. performed the EM sample set-up and imaging; C.R.M. performed the viral experiments; M.K. and L.M.B. performed the adhesion experiments; E.J.B. was involved in the collection of the FHS plasma data; E.M. conducted the statistical analysis of the FHS data; E.A.K.-J., K.R., and J.E.F. designed the experiments, interpreted the results, and contributed to the writing of the paper; all authors contributed to the editing of the manuscript; and J.E.F. oversaw the project.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Jane E. Freedman, University of Massachusetts Medical School, Albert Sherman Center, 368 Plantation St, S7-1051, Worcester, MA 01605; e-mail: Jane.Freedman@umassmed.edu.


This feature is available to Subscribers Only
Sign In or Create an Account Close Modal