Key Points
Ezh2 represses Ifng, Gata3, and Il10 loci in naïve CD4+T cells, and its deficiency leads to Th1 skewing and IL-10 overproduction in Th2 cells.
Ezh2 deficiency activates multiple death pathways in differentiated effector Th cells.
Differentiation of naïve CD4+ T cells into effector (Th1, Th2, and Th17) and induced regulatory (iTreg) T cells requires lineage-specifying transcription factors and epigenetic modifications that allow appropriate repression or activation of gene transcription. The epigenetic silencing of cytokine genes is associated with the repressive H3K27 trimethylation mark, mediated by the Ezh2 or Ezh1 methyltransferase components of the polycomb repressive complex 2 (PRC2). Here we show that silencing of the Ifng, Gata3, and Il10 loci in naïve CD4+ T cells is dependent on Ezh2. Naïve CD4+ T cells lacking Ezh2 were epigenetically primed for overproduction of IFN-γ in Th2 and iTreg and IL-10 in Th2 cells. In addition, deficiency of Ezh2 accelerated effector Th cell death via death receptor–mediated extrinsic and intrinsic apoptotic pathways, confirmed in vivo for Ezh2-null IFN-γ–producing CD4+ and CD8+ T cells responding to Listeria monocytogenes infection. These findings demonstrate the key role of PRC2/Ezh2 in differentiation and survival of peripheral T cells and reveal potential immunotherapeutic targets.
Introduction
Immune homeostasis depends on the regulated differentiation of naïve CD4+ T cells into effector T-helper (Th) and induced regulatory (iTreg) T-cell lineages.1 Effector Th cells protect against infections and tumors but may mediate autoimmune disease if their activation is unregulated2,3 or if they fail to die after activation.4,-6 iTreg cells are complementary to thymus-derived natural regulatory T (nTreg) cells7 and dampen excessive inflammation.8
The differentiation of Th cell lineages is initiated by activation of naïve CD4+ T cells in the appropriate cytokine milieu and determined by the lineage-specific transcription factors T-bet (Th1), GATA3 (Th2), and ROR-γt (Th17) in effector T cells and Foxp3 in iTreg. Th1, Th2, and Th17 cells secrete distinct cytokines—respectively, interferon-γ (IFN-γ), interleukin 4 (IL-4), and IL-17—which, in concert with lineage-specific transcription factors inhibit differentiation of the other T-cell lineages.9 For example, counter-regulation occurs between Th1 and Th2 lineages through T-bet, GATA-3, and STAT410,11 ; between Th1 and Th17 lineages through T-bet, Runx1, and ROR-γt12 ; and between effector Th and iTreg cells through T-bet, GATA3, ROR-γt, Runx1, and Foxp3.13,,-16 Th1, Th2, Th17, and iTreg cells also produce the anti-inflammatory cytokine IL-10, which is a crucial feedback regulator of diverse immune responses.17
Epigenetic regulation through DNA methylation, histone modification, and noncoding RNAs is essential to fine-tune gene expression and stabilize cell lineages18 during naïve CD4+ T-cell differentiation.19,20 The diversity of epigenetic modifications creates the potential for considerable redundancy at each gene locus. For histones, only a few core modifications seem to characterize genes as active or accessible, inactive but poised, or silent.21 Dimethylation or trimethylation of histone 3 lysine 9 (H3K9) or H3K27, often found in the heterochromatin or at discrete sites within active genes, can repress inappropriate transcription.20 Depletion of the methyltransferase SUV39H1 that mediates H3K9 trimethylation (H3K9me3) in Th2 cells results in transcription of the Th1 cytokine Ifng.22 Effector T-cell signature cytokine loci such as the Ifng locus in Th2 cells23,24 and the Il4 locus in Th1 cells25 are marked by repressive H3K27me3 marks. In Th2, Th17, and iTreg lineages, the Tbx21 locus encoding the Th1-cell transcription factor T-bet is “poised” for activation or repression by the combination of active H3K4me3 and repressive H3K27me3 marks.26 It was suggested that the Th2 transcription factors GATA3 and STAT6 recruit the polycomb methyltransferase Ezh2 to the Ifng promoter and that H3K27me3 is associated with repression of IFN-γ transcription in Th2 cells.27 However, it was also reported that recruitment of Ezh2 was required for both IFN-γ and IL-4 production in differentiated Th1 and Th2 cells.28
The polycomb repressive complex (PRC) proteins form 2 closely related complexes—PRC2 and PRC1. H3K27 trimethylation is catalyzed by the methyltransferases Ezh2 or Ezh1,29 which are core components of PRC2 along with Suz12 and Eed.30 PRC1 occupies some of the PRC2 sites31 and may function downstream of PRC2.32 Effector CD4+ T cells lacking the PRC1 proteins Bmil33 and Ring1B34 have impaired survival, at least in part because of activation of apoptosis mediated by Noxa and Bim. EZH2 overexpression and activating mutations are implicated in tumorigenesis and correlate with poor prognosis in several tumor types.35 This spurred the development of EZH2 inhibitors that, by inducing tumor cell growth arrest and cell death, show therapeutic promise in cancer.36 Previously, it was reported that cytosolic Ezh2 was required for actin polymerization after T-cell receptor (TCR) ligation.37 A role for Ezh2 in suppressing Th1 and Th2 cytokine production38 and cell death39 has recently been reported. It is not entirely clear whether Ezh2-PRC2 plays a role in H3K27me3 at cytokine loci in naïve CD4+ T cells and whether H3K27me3 has a nonredundant role in Th cell lineage differentiation and survival. Here, by analyzing the effects of T cell–specific Ezh2 deletion, we show that Ezh2-PRC2 plays a distinct, nonredundant role in regulating the fate of differentiating naïve CD4+ T cells.
Materials and methods
Methods regarding experimental mice; naïve CD4+ T-cell purification and differentiation; cell staining for flow cytometry analysis; infection with Listeria monocytogenes (LM); western blotting; RNA-seq analysis; gene-set testing and pathway analysis; ChIP-seq data analysis; ChiP-qPCR; human blood donors; and naïve CD4+ T-cell isolation, activation, and phenotyping are detailed in the supplemental Methods, available on the Blood Web site. Experimental protocols were approved by the Walter and Eliza Hall Institute Animal Ethics Committee and conducted in accordance with the Principles of Laboratory Animal Care (National Institutes of Health publication no. 85–23, revised 1985). RNA-seq and ChIP-seq data were deposited at National Center for Biotechnology Information Gene Expression Omnibus (GSE57500). Human studies were approved by the Human Research Ethics Committee of Melbourne Health and the Walter and Eliza Hall Institute of Medical Research, and blood samples were obtained with individual informed consent. Blood samples from healthy individuals were obtained through Volunteer Blood Donor Registry. Studies were conducted in accordance with the Declaration of Helsinki.
Results
Ezh2-null naïve CD4+ T cells display differentiation skewing and cell death upon differentiation
Ezh2fl/fl.CD4Cre mice were established by crossing Ezh2f1/f140 with CD4Cre transgenic mice.41 The effects of Ezh2 deletion on T-cell development and on the proportion and phenotype of peripheral naïve and memory CD4+ T cells are shown in supplemental Figure 1.We examined the differentiation of Th1, Th2, Th17, and iTreg lineages in vitro42 (Figure 1A) after sorting naïve CD4+ T cells (CD4+CD25–CD62Lhi CD44lo) directly from Ezh2fl/fl.CD4Cre mice or mice reconstituted with a mixture of Ezh2fl/fl.CD4Cre (Ly5.2+) and wild-type (WT) (Ly5.1+) bone marrow.
Differentiation of Ezh2-null naïve CD4+ T cells. (A) Schema of naïve CD4+ T-cell activation and differentiation conditions. (B) Four-day proliferation of naïve CD4+ T cells purified from Ezh2fl/fl and Ezh2fl/fl.CD4Cre mice that were cocultured with WT (Ly5.1+) naïve CD4+ T cells. iTreg-1 and -5 indicate the addition of 1 and 5 ng/mL TGF-β1 under iTreg conditions. Cytokine production in Th0 (D), Th2 (E), Th17 (E), and Th1 and iTreg (F) cells at day 4. (G) T-bet and Gata-3 mean fluorescent intensity (MFI) in Th0, Th1, and Th2 cells. Data are representative of 3 independent experiments for (A-G). (H) mRNA expression of Tbx21, Ifng, and Foxp3 in naïve and differentiated Th0 and iTreg cells; Rps9 was used as a reference gene. Data are presented as mean ± standard error of the mean (SEM) of 2 independent experiments.
Differentiation of Ezh2-null naïve CD4+ T cells. (A) Schema of naïve CD4+ T-cell activation and differentiation conditions. (B) Four-day proliferation of naïve CD4+ T cells purified from Ezh2fl/fl and Ezh2fl/fl.CD4Cre mice that were cocultured with WT (Ly5.1+) naïve CD4+ T cells. iTreg-1 and -5 indicate the addition of 1 and 5 ng/mL TGF-β1 under iTreg conditions. Cytokine production in Th0 (D), Th2 (E), Th17 (E), and Th1 and iTreg (F) cells at day 4. (G) T-bet and Gata-3 mean fluorescent intensity (MFI) in Th0, Th1, and Th2 cells. Data are representative of 3 independent experiments for (A-G). (H) mRNA expression of Tbx21, Ifng, and Foxp3 in naïve and differentiated Th0 and iTreg cells; Rps9 was used as a reference gene. Data are presented as mean ± standard error of the mean (SEM) of 2 independent experiments.
In differentiation assays, naïve CD4+ T cells from Ezh2fl/fl control or Ezh2fl/fl.CD4Cre mice were labeled with cell-tracer violet dye and mixed with WT (Ly5.1+) naïve cells at a 1:1 ratio to assess their proliferation and survival after activation. By day 4, control and Ezh2fl/fl.CD4Cre naïve CD4+ T cells had proliferated similarly (Figure 1B) and comprised similar proportions of live cells (supplemental Figure 2A). However, by day 6, the proportion of Ezh2fl/fl.CD4Cre effector (Th0, Th1, Th2, Th17) cells, but not iTreg cells, had decreased (supplemental Figure 2B), suggesting increased cell death in these effector lineages. We then characterized Th0, Th1, Th2, and Th17 differentiation at day 4 and iTreg differentiation at days 4 to 6. Under nonpolarizing Th0 conditions, the proportions of cells expressing the Th1 cytokine IFN-γ and the Th2 cytokines IL-4, IL-5, and IL-13 were increased in Ezh2fl/fl.CD4Cre cells. IL-10, a feedback regulator of Th1 and Th2 responses, was also dramatically increased in Th0 cells (Figure 1C). The production of IL-4, IL-5, and IL-13 was increased in Th2 cells, which also had the most prominent upregulation of IL-10 (Figure 1D). Based on IL-17A expression, Th17 cell differentiation was similar between control and Ezh2fl/fl.CD4Cre naïve CD4+ T cells (Figure 1E). Under both Th1 and iTreg conditions, IFN-γ overproduction was observed (Figure 1F). Ezh2fl/fl.CD4Cre Th0 and Th2 cells had increased expression of T-bet and Gata-3 proteins (Figure 1G). Tbx21 and Ifng mRNA were significantly upregulated in Ezh2fl/fl.CD4Cre cells, and expression of Foxp3 was decreased (Figure 1H). Apart from IFN-γ, IL-4 and IL-17A were not detected in Ezh2fl/fl.CD4Cre Th1 and iTreg cells.
IFN-γ blockade reverses skewed differentiation of Ezh2-null naïve CD4+ T cells
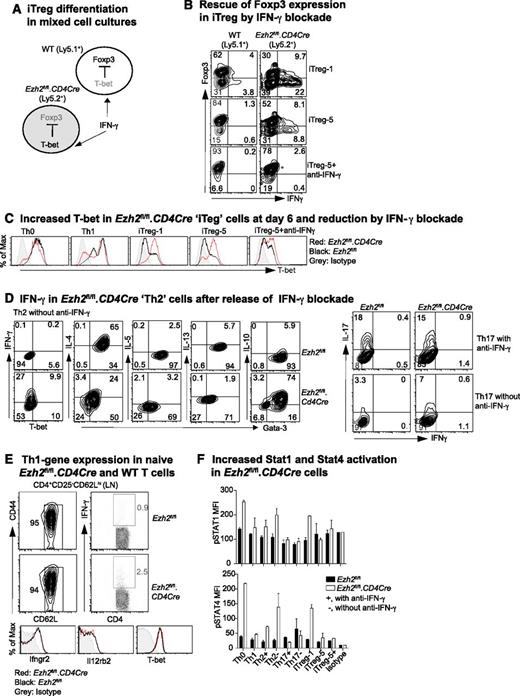
IFN-γ promotes Th1-cell differentiation by activating STAT1, leading to the transcription of Tbx21 and the IL-12 receptor β2 (Il12rb2), with subsequent activation of STAT4 by IL-12.43 To investigate how IFN-γ limits Foxp3 expression in iTreg cells, we co-cultured WT (Ly5.1+) and Ezh2fl/fl.CD4Cre (Ly5.2+) naïve CD4+ T cells purified from bone marrow–reconstituted chimeric mice under iTreg differentiation conditions (Figure 2A). In mixed cultures, Ezh2fl/fl.CD4Cre, but not WT, cells exhibited increased IFN-γ production and decreased Foxp3 expression in keeping with intrinsic dysfunction. However, in the presence of IFN-γ–blocking antibody, Ezh2fl/fl.CD4Cre cells regained Foxp3 expression (Figure 2B) and decreased their high T-bet expression at day 6 (Figure 2C). Thus, impaired differentiation of Ezh2fl/fl.CD4Cre naïve CD4+ T cells into iTreg was secondary to the autocrine effect of IFN-γ, consistent with previous evidence13 that IFN-γ inhibits Foxp3 expression by activating STAT1 and T-bet.
IFN-γ blockade prevents impaired differentiation of Ezh2-null naïve CD4+ T cells. (A) Schema of mixed-cell culture conditions. (B) iTreg differentiation in mixed-cell cultures as illustrated in (A) in the presence or absence of IFN-γ–blocking antibody. (C) Expression of T-bet at day 6 in differentiated cells. (D) Cytokine production in Th2 and Th17 cells in the presence or absence of IFN-γ blockade. (E) IFN-γ production by naïve CD4+ T cells activated by phorbol myristate acetate (PMA)+ionomycin for 5 hours, and expression of IFN-γ receptor (Ifngr2), IL-12 receptor (Il12rb2), and T-bet genes in naïve CD4+ T cells. (F) Activation of STAT1 and STAT4 in 4-day differentiated T cells in the presence (+) of absence (–) of IFN-γ blockade when restimulated by PMA+ionomycin for 5 hours. Results shown are mean ± SEM of duplicates, representative of 3 independent experiments.
IFN-γ blockade prevents impaired differentiation of Ezh2-null naïve CD4+ T cells. (A) Schema of mixed-cell culture conditions. (B) iTreg differentiation in mixed-cell cultures as illustrated in (A) in the presence or absence of IFN-γ–blocking antibody. (C) Expression of T-bet at day 6 in differentiated cells. (D) Cytokine production in Th2 and Th17 cells in the presence or absence of IFN-γ blockade. (E) IFN-γ production by naïve CD4+ T cells activated by phorbol myristate acetate (PMA)+ionomycin for 5 hours, and expression of IFN-γ receptor (Ifngr2), IL-12 receptor (Il12rb2), and T-bet genes in naïve CD4+ T cells. (F) Activation of STAT1 and STAT4 in 4-day differentiated T cells in the presence (+) of absence (–) of IFN-γ blockade when restimulated by PMA+ionomycin for 5 hours. Results shown are mean ± SEM of duplicates, representative of 3 independent experiments.
The presence of IFN-γ–blocking antibody, normally required to permit differentiation of Th2 and Th17 cells (Figure 1A), could have masked an effect of Ezh2 deficiency on Th2 (as shown in Figure 1D) and Th17 differentiation. We then examined differentiation in the absence of IFN-γ–blocking antibody. Under Th2 conditions, a substantial proportion of Ezh2fl/fl.CD4Cre cells expressed IFN-γ and had reduced expression of Gata-3 and IL-4, although their expression of IL-10 was sustained (Figure 2D). Under Th17 conditions, differentiation was unchanged (Figure 2D, right panel), possibly because of the antagonistic effects of IL-644 and/or transforming growth factor (TGF)-β45 to suppress Th1 differentiation. Overall, these results show that Ezh2 suppresses IFN-γ expression, permitting Th2 and iTreg differentiation.
In mixed cultures, Foxp3 expression in WT cells exposed to IFN-γ from Ezh2fl/fl.CD4Cre cells remained largely uncompromised. This suggested that the absence of Ezh2 was associated with intrinsic effects, leading to an increased responsiveness to IFN-γ. However, naïve CD4+ T cells from WT and Ezh2fl/fl.CD4Cre mice had similar expression of the IFN-γ receptor (Ifngr2), IL-12 receptor (Il12rb2), and T-bet. In addition, the vast majority (>95%) of Ezh2fl/fl.CD4Cre naïve CD4+ T cells did not produce IFN-γ when activated by PMA and ionomycin over a short 5-hour period insufficient for the expression of newly transcribed cytokine genes (Figure 2E). This suggested a requirement for prolonged T-cell activation for increased expression of genes, leading to skewed Th1 differentiation. Under differentiation conditions at day 4, Ezh2fl/fl.CD4Cre cells had higher STAT1 and STAT4 phosphorylation when they were re-stimulated by PMA and ionomycin for 5 hours in the absence of IFN-γ–blocking antibody (Figure 2F), demonstrating that excessive IFN-γ production promoted activation of genes for Th1 differentiation.
Suppression of IFN-γ production by Ezh2 requires other PRC2 components
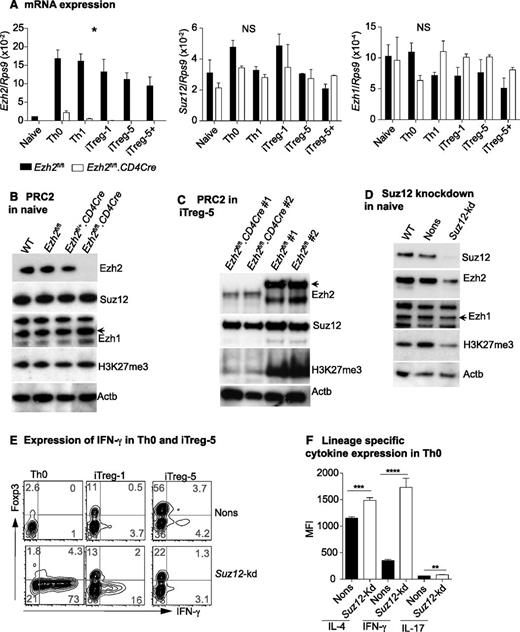
To gain insight into how Ezh2 regulates IFN-γ production, we examined expression of the PRC2 components during T-cell differentiation. Ezh2 transcription was dramatically increased upon T-cell activation and differentiation, but in the absence of Ezh2, transcription of Ezh1 and Suz12 was unchanged (Figure 3A). In naïve CD4+ T cells, expression of Ezh1 and Suz12 protein, and global H3K27me3, was comparable in Ezh2fl/fl.CD4Cre and control cells (Figure 3B). In WT cells, activation and differentiation increased the expression of PRC2 components and decreased global H3K27 trimethylation (supplemental Figure 3). In iTreg cells, loss of Ezh2 resulted in a further marked global reduction of the repressive H3K27me3 marks (Figure 3C). Ezh2fl/+.CD4Cre cells had similar Ezh2 expression compared with WT or Ezh2fl/fl controls (see Figure 3B), and we did not observe significant differences in iTreg cell differentiation in these cells (data not shown). Taken together, these findings indicate that Ezh2 is not required for the expression or maintenance of PRC2 components and is not essential to maintain global H3K27 trimethylation in naïve CD4+ T cells. However, after proliferation and differentiation, Ezh2 has a clear role in global H3K27me3.
Suppression of IFN-γ production by Ezh2 requires PRC2. (A) Expression of Ezh2, Ezh1, and Suz12 in naïve and differentiated T cells relative to Rps9. Data are mean ± SEM of 2 independent experiments. P values were calculated from 2-way analysis of variance (*P < .05). (B) Western blot showing Ezh2, Suz12, Ezh1, H3k27me3, and Actb in naïve CD4+ T cells isolated from WT (Ly5.1), Ezh2fl/fl, Ezh2fl/+CD4Cre, and Ezh2fl/fl.CD4Cre mice. (C) Western blots of iTreg cells. (D) Western blot of naïve CD4+ T cells that are depleted of Suz12. (E) IFN-γ and Foxp3 expression in Suz12-kd Th0 and iTreg cells. (F) Cytokine expression in Suz12-kd Th0 cells.
Suppression of IFN-γ production by Ezh2 requires PRC2. (A) Expression of Ezh2, Ezh1, and Suz12 in naïve and differentiated T cells relative to Rps9. Data are mean ± SEM of 2 independent experiments. P values were calculated from 2-way analysis of variance (*P < .05). (B) Western blot showing Ezh2, Suz12, Ezh1, H3k27me3, and Actb in naïve CD4+ T cells isolated from WT (Ly5.1), Ezh2fl/fl, Ezh2fl/+CD4Cre, and Ezh2fl/fl.CD4Cre mice. (C) Western blots of iTreg cells. (D) Western blot of naïve CD4+ T cells that are depleted of Suz12. (E) IFN-γ and Foxp3 expression in Suz12-kd Th0 and iTreg cells. (F) Cytokine expression in Suz12-kd Th0 cells.
Ezh2 was previously reported to have a PRC2-independent role under certain experimental conditions.46 To determine whether Ezh2 functions within the context of PRC2, we depleted the nonredundant PRC2 component Suz12 by retroviral-mediated shRNA knockdown. In naïve CD4+ T cells, this resulted in a global reduction of H3K27me3 (Figure 3D), which was in contrast to Ezh2 depletion (Figure 3B). Similarly to Ezh2fl/fl.CD4Cre cells, Suz12-depleted naïve CD4+ T cells also exhibited increased IFN-γ and decreased Foxp3 expression under iTreg differentiation conditions (Figure 3E). In Th0 cells, expression of all lineage-specific cytokines was significantly increased in Suz12-kd compared with nonsilencing (Nons) controls (Figure 3F). Of note, we also observed a decrease in the expression of Ezh2 in naïve CD4+ T cells depleted of Suz12 (Figure 3D). These results further confirm that PRC2 suppresses Th1 and Th2 cytokine gene expression in nonpolarized conditions, most likely dependent on Ezh2 in the context of PRC2.
Association of genes regulated by Ezh2 with trimethylation of histone 3 lysine 27
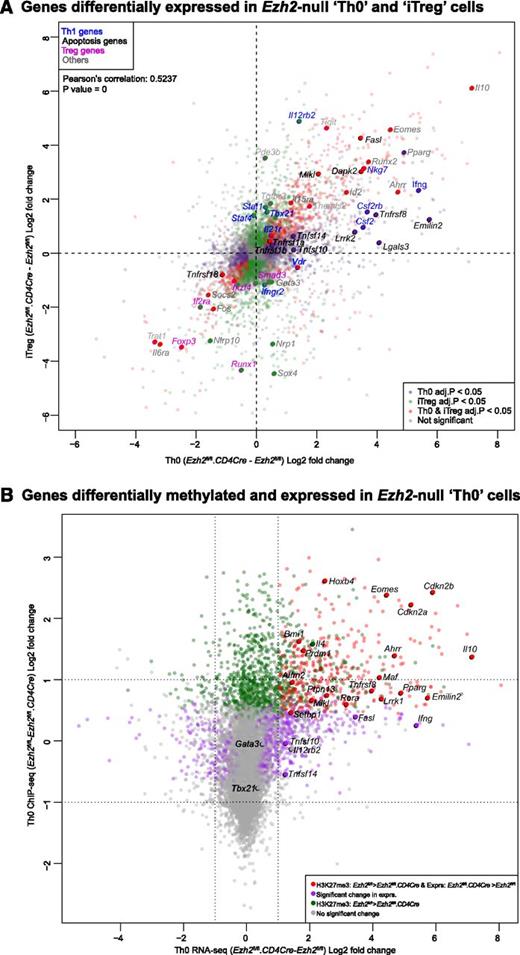
We performed RNA-seq to identify genes regulated by Ezh2 during T-cell activation and differentiation. Loss of Ezh2 altered the expression of 1328 genes in Th0 (supplemental Figure 4A and supplemental Table 1) and 1979 genes in iTreg cells (supplemental Figure 4B and supplemental Table 2). Gene expression changes were positively correlated in both cell types (Figure 4A), indicating that Ezh2 targets similar genes in these cells. As expected, Ifng was one of the genes most increased in expression after loss of Ezh2. In addition, expression of Tbx21 homolog Eomes, a transcription factor that regulates IFN-γ production,38 was also significantly increased.
Association of genes regulated by Ezh2 and trimethylation of histone 3 lysine 27. (A) Gene expression in Ezh2fl/fl.CD4Cre Th0 vs iTreg cells, relative to Ezh2fl/fl cells. Each point represents a single gene. Red dots denote genes significantly differentially expressed (FDR adjusted P < .05) in both Th0 and iTreg cells; green dots denote genes only differentially expressed in iTreg cells; purple dots denote genes only differentially expressed in Th0 cells. Selected genes belonging to relevant gene sets are named. (B) Change in expression in Ezh2fl/fl.CD4Cre Th0 cells relative to Ezh2fl/fl vs change in H3K27me3 of Ezh2fl/fl Th0 cells relative to Ezh2fl/fl.CD4Cre. Each point represents a single gene. Red dots denote genes significantly less methylated in Ezh2fl/fl.CD4Cre and upregulated; green dots denote genes significantly less methylated in Ezh2fl/fl.CD4Cre but not differentially expressed; purple dots denote genes significantly differentially expressed with no change in H3Kme3; gray dots denote genes that do not show a significant change in either expression or H3K27me3. (C-D) ChIP-qPCR showing H3K27me3 and H3K4me3 marks at Th1, Th2, and control loci. Data shown are mean ± SEM of 3 independent experiments (paired Student t test: *P < .05, **P < . 01).
Association of genes regulated by Ezh2 and trimethylation of histone 3 lysine 27. (A) Gene expression in Ezh2fl/fl.CD4Cre Th0 vs iTreg cells, relative to Ezh2fl/fl cells. Each point represents a single gene. Red dots denote genes significantly differentially expressed (FDR adjusted P < .05) in both Th0 and iTreg cells; green dots denote genes only differentially expressed in iTreg cells; purple dots denote genes only differentially expressed in Th0 cells. Selected genes belonging to relevant gene sets are named. (B) Change in expression in Ezh2fl/fl.CD4Cre Th0 cells relative to Ezh2fl/fl vs change in H3K27me3 of Ezh2fl/fl Th0 cells relative to Ezh2fl/fl.CD4Cre. Each point represents a single gene. Red dots denote genes significantly less methylated in Ezh2fl/fl.CD4Cre and upregulated; green dots denote genes significantly less methylated in Ezh2fl/fl.CD4Cre but not differentially expressed; purple dots denote genes significantly differentially expressed with no change in H3Kme3; gray dots denote genes that do not show a significant change in either expression or H3K27me3. (C-D) ChIP-qPCR showing H3K27me3 and H3K4me3 marks at Th1, Th2, and control loci. Data shown are mean ± SEM of 3 independent experiments (paired Student t test: *P < .05, **P < . 01).
To identify gene networks regulated by Ezh2, we performed competitive gene set testing using curated gene sets from the Broad Institute Molecular Signatures Database. Gene sets associated with PRC2, interferon responsive genes, and apoptosis were significantly more prominent in Ezh2fl/fl.CD4Cre Th0 cells, whereas targets of Smarca2/4 (part of the SWI/SNF complex, antagonistic to PRC2) were less prominent. Gene sets differentially expressed in Ezh2fl/fl.CD4Cre iTreg cells largely overlapped those in Th0 cells, with the exception of Foxp3 targets and cell death–related gene sets (supplemental Table 3), consistent with reduced Foxp3 expression and lack of evidence for cell death in Ezh2fl/fl.CD4Cre iTreg cells. KEGG pathway analysis revealed that autoimmune/inflammatory pathways (type 1 diabetes mellitus, autoimmune thyroid disease, graft-vs-host disease, allograft rejection, and asthma) were over-represented in both Ezh2fl/fl.CD4Cre Th0 and iTreg cells (supplemental Table 4), in accord with the marked increase in IFN-γ production after activation of naïve Ezh2fl/fl.CD4Cre CD4+ T cells.
To determine to what extent Ezh2 uses H3K27 trimethylation to regulate gene expression during T-cell differentiation, we compared our data with publicly available H3K27me3 ChIP-seq data from naïve CD4+ T and iTreg cells.26 This revealed that genes upregulated in Ezh2fl/fl.CD4Cre Th0 and iTreg cells were more likely to have been marked at their promoters by H3K27me3 (supplemental Figure 5), consistent with its role in repressing gene transcription and with a role for Ezh2 in effecting this epigenetic modification. We then performed H3K27me3 ChIP-seq on Ezh2fl/fl and Ezh2fl/fl.CD4Cre Th0 cells. Consistent with cellular phenotype and RNA-seq data, we observed a loss of the H3K27me3 at Eomes, Il4, and Il10 loci (Figure 4B and supplemental Figure 6). Very low levels of H3K27me3 marks were present at Ifng and Tbx21 loci in differentiated Ezh2fl/fl Th0 cells (data not shown), suggesting that, upon differentiation, an increase or activation of transcription factors accounts for IFN-γ overproduction. A significant loss of H3K27me3 was observed >2 kb upstream of the Gata3 locus (supplemental Figure 6), but this did not result in increased transcription (Figure 4A-B). Of the 22 381 genes tested for changes in H3K27me3, 1360 showed a statistically significant decrease in Ezh2fl/fl.CD4Cre Th0 cells compared with Ezh2fl/fl. Furthermore, 404 of these genes also showed a concomitant increase in expression in Ezh2fl/fl.CD4Cre Th0 cells (supplemental Table 5), suggesting that these loci are likely direct Ezh2-PRC2 targets.
We also examined whether Ezh2 is required for H3K27me3 at the Th1 and Th2 loci before and after naïve CD4+ T-cell differentiation into Th1 and Th2 cells, along with association of the active histone mark H3K4me3. In Ezh2fl/fl naïve CD4+ T cells, H3K27me3 was enriched across the Ifng locus and was significantly reduced in the absence of Ezh2. Loss of H3K27me3 occurred when naïve CD4+ T cells differentiated into Th1 or Th2 cells. In Th2 cells, Tbx21 and Eomes promoter H3K27me3 was dependent on Ezh2. Active H3K4me3 marks were enriched at theTh1 loci in naïve and differentiated cells, with the exception of the Ifng promoter in Th2 cells (Figure 4C). In the absence of Ezh2, significant loss of H3K27me3 was observed in the Gata3 and Il10 promoters in naïve, in the Il4 promoter in Th1, and in the Il10 promoter in Th2 cells. H3K4me3 marks were also enriched at the Th2 loci, except at the Il4 promoter in Th1 cells (Figure 4D). These results suggest that Th1 and Th2 loci in naïve CD4+ T cells are marked with repressive and active histone marks, and that Ezh2 has a nonredundant role in H3K27me3 at specific loci before naïve cell differentiation.
Ezh2 differentially controls cell survival of effector Th and iTreg cells
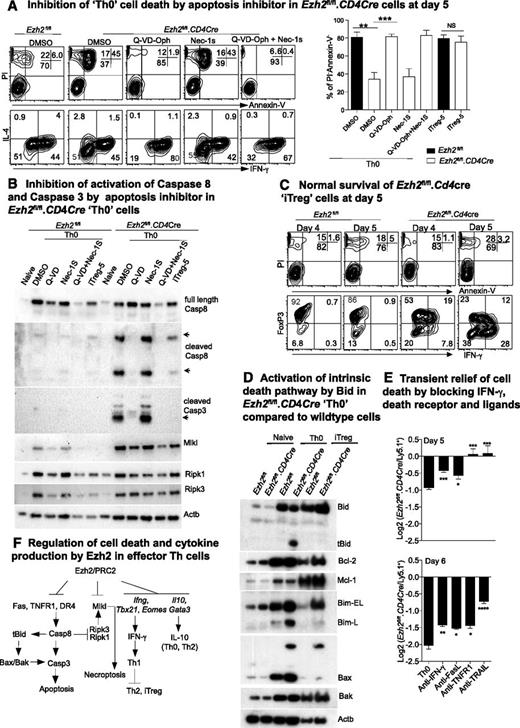
The number of Ezh2fl/fl.CD4Cre effector Th but not iTreg cells decreased dramatically compared with control cells by 6 days after differentiation (supplemental Figure 1B), suggesting that cell death pathways were differentially activated in Ezh2fl/fl.CD4Cre Th cells. MLKL is a pseudokinase required to induce necroptosis.47,48 Intriguingly, we observed an increase in transcription (Figure 4A), loss of H3K27me3 (Figure 4B), and expression (Figure 5B) of MLKL in both Ezh2fl/fl.CD4Cre Th0 and iTreg cells. Furthermore IFN-γ has been implicated in RIPK1/RIPK3-mediated necroptotic cell death in Fadd−/− cells.49 We therefore tested whether Ezh2fl/fl.CD4Cre Th0 cells died by necroptosis, by treating Ezh2fl/fl.CD4Cre naïve CD4+ T cells with the RIPK1 inhibitor Nec-1s. Nec-1s provided no protection (Figure 5A). Alternatively, Q-VD-Oph, a pan-caspase inhibitor, significantly increased the proportion of live (PI–ve, AnnexinV–ve) Th0 cells compared with sham-treated cells (Figure 5A). Q-VD-Oph also increased survival of the other Ezh2fl/fl.CD4Cre effector Th-cell lineages (data not shown). Activated caspase-8 and caspase-3 were observed in Ezh2fl/fl.CD4Cre Th0 cells and were absent in cells treated with Q-VD-Oph, but not Nec-1s (Figure 5B). Consistent with the cell number observations at day 6 post-differentiation (supplemental Figure 1B), activated caspases were not substantially increased in Ezh2fl/fl.CD4Cre iTreg-5 compared with WT cells, and loss of Ezh2 did not affect the viability of iTreg cells at 4 or 5 days postdifferentiation (Figure 5C). Furthermore caspase-cleaved and -activated tBid was present in Ezh2fl/fl.CD4Cre Th0 cells but not in Ezh2fl/fl or iTreg cells, consistent with involvement of the Bax/Bak-dependent intrinsic death pathway1 (Figure 5D).
Ezh2 differentially controls cell survival of effector and regulatory T cells. (A) Live cell and cytokine staining in Th0 cells at day 5 in the presence of apoptosis or necrosis inhibitors (left panel). Percentages of live cells in 3 independent experiments (unpaired Student t test: **P < .01, ***P < .001) (right panel). (B) Western blot of death receptor–related cell death proteins in Th0 and iTreg cells from Ezh2fl/fl and Ezh2fl/fl.CD4Cre cells. (C) Live cell and cytokine staining in iTreg cells at days 4 to 5. (D) Western blot of intrinsic cell death proteins in naïve Th0 and iTreg cells from Ezh2fl/fl and Ezh2fl/fl.CD4Cre cells. (E) Log2 ratio of live Ezh2fl/fl.CD4Cre and WT cells at days 5 and 6 in mixed-cell cultures under Th0 conditions. (F) Model summary of regulation of IFN-γ production and cell death by Ezh2 in effector Th cells.
Ezh2 differentially controls cell survival of effector and regulatory T cells. (A) Live cell and cytokine staining in Th0 cells at day 5 in the presence of apoptosis or necrosis inhibitors (left panel). Percentages of live cells in 3 independent experiments (unpaired Student t test: **P < .01, ***P < .001) (right panel). (B) Western blot of death receptor–related cell death proteins in Th0 and iTreg cells from Ezh2fl/fl and Ezh2fl/fl.CD4Cre cells. (C) Live cell and cytokine staining in iTreg cells at days 4 to 5. (D) Western blot of intrinsic cell death proteins in naïve Th0 and iTreg cells from Ezh2fl/fl and Ezh2fl/fl.CD4Cre cells. (E) Log2 ratio of live Ezh2fl/fl.CD4Cre and WT cells at days 5 and 6 in mixed-cell cultures under Th0 conditions. (F) Model summary of regulation of IFN-γ production and cell death by Ezh2 in effector Th cells.
Although we did not observe significant H3K27me3 changes in death receptors and ligands in Ezh2fl/fl.CD4Cre Th0 cells (data not shown), expression of Fasl, Tnfr1a, and Trail (Tnfsf10) was increased (Figure 4A). To determine whether death receptor signaling pathways might be responsible for activating caspase-8 in these cells, we cocultured Ezh2fl/fl.CD4Cre and WT cells purified from chimeric mice at a 1:1 ratio under Th0 conditions in the presence of blocking antibodies to IFN-γ, FasL, TNFR1, or TRAIL. TNFR1 or TRAIL blockade completely prevented Ezh2fl/fl.CD4Cre Th0 cell death at day 5, and IFN-γ or FasL blockade provided a small but significant survival advantage (Figure 5E). The effects of blocking antibodies persisted into day 6, but cell death was not completely prevented (Figure 5E). Thus, in parallel with T helper–cell differentiation skewing, multiple death pathways appeared to be activated in Ezh2-null effector CD4+ T cells (Figure 5F).
Ezh2 controls differentiation and survival of effector Th cells in vivo
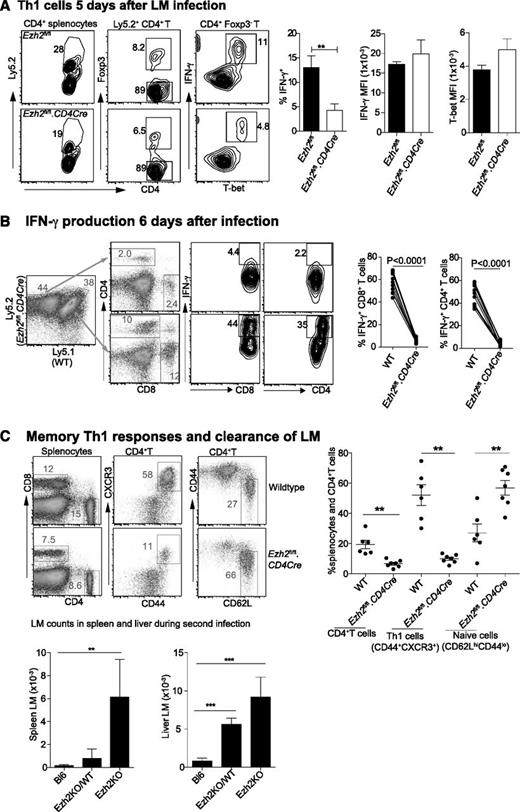
We then investigated the effect of Ezh2 on T-cell differentiation and survival in vivo. Mixed bone marrow chimeric mice containing WT (Ly5.1+) and either Ezh2fl/fl.CD4Cre (Ly5.2+) or Ezh2fl/fl cells were injected intravenously (IV) with 2 × 104 LM, which predominantly induces a Th1 response.50 Analysis of spleen cells harvested from 5 day–infected mice showed that the proportion of IFN-γ+ T-bet+ Th1 cells was significantly reduced in Ezh2fl/fl.CD4Cre compared with Ezh2fl/fl control mice, although their expression of T-bet and IFN-γ was similar (Figure 6A). Six days post-LM infection, we observed a further reduction in Ezh2-null IFN-γ–producing CD4+ and CD8+ T cells in chimeric mice (Figure 6B). To examine whether enhanced Th1 cell death leads to impaired LM clearance, we injected 1 × 104 LM IV into WT (Bl6) mice, chimeric mice harboring 70% Ezh2fl/fl.CD4Cre and 30% WT splenocytes (Ezh2KO/WT), or Ezh2fl/fl.CD4Cre bone marrow–reconstituted mice (Ezh2KO), followed by challenge with 2 × 105 LM IV 4 weeks later. Three days post-secondary infection splenocytes showed no differences in the total cell numbers (data not shown), but the Ezh2fl/fl.CD4Cre compartment had a significantly reduced proportion of memory CD4+ T cells. This was in contrast to the strong Th1 (CD44+CXCR3+) phenotype in the WT compartment. Mice reconstituted with Ezh2fl/fl.CD4Cre bone marrow failed to clear LM infection (Figure 6C).
Ezh2 controls differentiation and survival of effector T cells in vivo. Chimera mice (n = 5-8/group) were IV injected with 2 × 104 LM. Results shown are (A) CD4+ T cells that are IFN-γ producing Th1 cells 5 days postinfection; and (B) IFN-γ producing CD4+ and CD8+ T cells 6 days postinfection. (C) Four weeks after primary infection with 1 × 104 LM, mice were rechallenged with 2 × 105 LM. The phenotype of spleen CD4+ T cells and the bacterial number in spleen and liver lysates were determined 3 days after the second infection. Results shown are from 3 independent experiments. Unpaired Student t test: **P < .01.
Ezh2 controls differentiation and survival of effector T cells in vivo. Chimera mice (n = 5-8/group) were IV injected with 2 × 104 LM. Results shown are (A) CD4+ T cells that are IFN-γ producing Th1 cells 5 days postinfection; and (B) IFN-γ producing CD4+ and CD8+ T cells 6 days postinfection. (C) Four weeks after primary infection with 1 × 104 LM, mice were rechallenged with 2 × 105 LM. The phenotype of spleen CD4+ T cells and the bacterial number in spleen and liver lysates were determined 3 days after the second infection. Results shown are from 3 independent experiments. Unpaired Student t test: **P < .01.
EZH2 controls IFN-γ production and the survival of human naïve CD4+ T cells
IFN-γ plays a critical role in the pathogenesis of many autoimmune diseases,51 and relative deficiency of IFN-γ characterizes some chronic infections and tumor tolerance in humans.52 To examine the function of EZH2 in human CD4+ T cells, we used GSK-126, a highly selective inhibitor of EZH2 methyltransferase.53 GSK-126 significantly increased IFN-γ production in activated naïve CD4+ T cells (Th0) and decreased the expression of FOXP3 in iTreg cells (Figure 7A-C). By day 6, we observed significantly reduced numbers of both Th0 and iTreg cells after treatment with GSK-126 (Figure 7D). These effects were accompanied by a decrease in EZH2 and in global H3K27me3 expression in some but not all donors (Figure 7E), suggesting population heterogeneity in EZH2 expression or response to inhibition.
EZH2 suppresses IFN-γ production and death of human CD4+ T cells. (A) IFN-γ and FOXP3 expression in human Th0 and iTreg cells in the absence or presence of EZH2 inhibitor GSK-126. (B) Proportion of IFN-γ Th0 cells and (C) FOXP3 iTreg cells with or without EZH2 inhibitor GSK-126 during naïve cell differentiation in healthy individuals. (D) Fold change in day 6 differentiated cell number compared with day 0 naïve CD4+ T-cell input. Paired Student t test: 2-tailed for (B-D). (E) Expression of PRC2 components during naïve CD4+ T-cell differentiation.
EZH2 suppresses IFN-γ production and death of human CD4+ T cells. (A) IFN-γ and FOXP3 expression in human Th0 and iTreg cells in the absence or presence of EZH2 inhibitor GSK-126. (B) Proportion of IFN-γ Th0 cells and (C) FOXP3 iTreg cells with or without EZH2 inhibitor GSK-126 during naïve cell differentiation in healthy individuals. (D) Fold change in day 6 differentiated cell number compared with day 0 naïve CD4+ T-cell input. Paired Student t test: 2-tailed for (B-D). (E) Expression of PRC2 components during naïve CD4+ T-cell differentiation.
Discussion
H3K27me3 by PRC2 in differentiated Th cells is associated with gene repression and reflects the cellular context. Thus, H3K27me3 is enriched at T-cell signature cytokine loci, which specify opposing T-cell lineages (eg, at the Ifng locus in Th2 cells23,24 and Il4 locus in Th1 cells).25 In naïve CD4+ T cells, we found that the lack of PRC2, after depletion of its nonredundant subunit Suz12, diminished global H3K27me3. Ezh2 was required for global H3K27me3 in differentiated Th cells but not in naïve CD4+ T cells, a function potentially compensated for by Ezh1.29 We found that H3K27me3 at particular loci, including Ifng, Gata3, and Il10, is specifically dependent on PRC2-Ezh2 in naïve CD4+ T cells, consistent with cellular phenotype in neutrally activated Th0 cells. The absence of H3K27me3 mark at Ifng locus in naïve and at Eomes and Tbx21 loci in differentiated Th cells was permissive for IFN-γ overproduction, with resultant skewing of T-cell differentiation toward the Th1 lineage under Th2 and iTreg conditions. This was confirmed by IFN-γ blockade, which normalized differentiation of Ezh2-null naïve CD4+ T into Th2 and iTreg lineages. Loss of H3K27 trimethylation at Ifng locus in Ezh2-null naïve CD4+ T cells could bypass the requirement of T-bet–mediated chromatin remodeling,54 further permitting the access of transcription factors including T-bet and Eomes required for IFN-γ transcription in differentiated Th lineages.38 We did not observe IFN-γ overproduction in Ezh2-null cells under Th17 differentiation conditions, with or without IFN-γ blockade, which suggests that exposure to IL-644 and/or TGF-β45 blocks Th1 differentiation pathways, obviating the loss of H3K27me3.
Similar to Ezh2-null, Suz12-depleted naïve CD4+ T cells had significantly increased expression of IL-4 in Th0 cells. These results are consistent with PRC2 repressing via H3K27me3 at the Gata3 locus in naïve CD4+ T cells and at the Il4 locus in Th0 cells. However, when Th2 cells were differentiated in the absence of IFN-γ blocking antibody, the production of Th2 cytokines was reduced in Ezh2-null cells, likely because of the antagonistic effects of T-bet and STAT4 on GATA-3 expression.10,11 The production of the antiinflammatory cytokine IL-10 in Ezh2fl/fl.CD4Cre Th0 and Th2 cells could result from the loss of H3K27me3 at the Il10 locus in naïve CD4+ T and Th2 cells and the expression of the transcription factor Gata-355 in these cell types. A recent study by Tumes et al38 demonstrated an enhanced asthmalike pathology when recipient mice were transferred with Ezh2-null Th2 cells differentiated in vitro and that aged Ezh2-null mice had enriched effector memory Th2 cells. It was unclear whether these Th2 cells underwent cell death and produced IL-10 and IFN-γ transferred in vivo physiological impact of Ezh2 deficiency may depend on the models studied.
We demonstrated that Ezh2 maintains the survival of effector Th0 cells by suppression of extrinsic and intrinsic death receptor pathways. This is consistent with a recent study39 in which donor Ezh2-null effector T cells initially expanded but then underwent apoptotic death (as we observed in vitro and in vivo) and failed to elicit graft-versus-host disease in recipient mice. Cell death associated with loss of PRC2-Ezh2 may not be a result of direct loss of H3K27me3 at death gene loci and or rescued by single-gene deletion. For example, depletion of Bim was insufficient to rescue Ezh2-mediated cell death.39 Cell death is unlikely to be a consequence of excessive IFN-γ production because (1) significant cell death was observed in Th2 and Th17 cells under differentiation conditions that did not result in IFN-γ production; and (2) Q-VD-Oph rescued Ezh2fl/fl.CD4Cre Th0 cell death without altering IFN-γ production. Future experiments are required to identify potential transcription factors that enhance the expression of death receptors and/or their ligands. The role of PRC2-Ezh2 in governing differentiation and survival of T cells has therapeutic implications. EZH2 inhibitors induce cancer cell growth arrest and cell death36 but may also promote IFN-γ–dependent antitumor immunity if a means could be found to prolong cell survival. Conversely, agents that promote EZH2 function may suppress Th1-dependent autoimmune disease.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
We thank the Walter and Eliza Hall Institute Volunteer Blood Donor Registry for organizing volunteer blood donors. Dr Lorraine O’Reilly kindly provided antibodies for cell death analysis.
This work was supported by grants from the Australian National Health and MedicalResearch Council (305500 [L.C.H.] and 1016647 [J.-G.Z.]), and the Juvenile Diabetes Research Foundation (17-2013-547 [L.C.H.]).
L.C.H. is a Senior Principal Research Fellow, A.O. is a Career Development Fellow of the National Health and Medical Research Council, and M.E.B. is a Queen Elizabeth II Fellow of the Australian Research Council.
This work was made possible through Victorian State Government Operational Infrastructure Support and Australian National Health and Medical Research Council Research Institute Infrastructure Support Scheme.
Authorship
Contribution: Y.Z., S.K., M.E.B., and L.C.H. designed the experiments; Y.Z., S.K., E.B., M.T., G.N., J.-G.Z., and Y.Z. performed the experiments; J.M. and A.O. performed the bioinformatics analysis; Y.Z. and L.C.H. wrote the manuscript with input from J.M., A.M.L., S.K., J.S., and M.E.B.; and all authors contributed in discussions.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Leonard C. Harrison, Walter and Eliza Hall Institute of Medical Research, 1 G Royal Parade, Parkville, 3052, Victoria, Australia; e-mail: harrison@wehi.edu.au.
References
Author notes
M.E.B. and L.C.H. are cosenior authors.




This feature is available to Subscribers Only
Sign In or Create an Account Close Modal