To the editor:
Despite considerable progress of chemotherapeutic strategies and the introduction of the proteasome inhibitor bortezomib, multiple myeloma (MM) remains an incurable disease.1 Mutations or loss of p53 occur in roughly 10% of untreated MM cells and are closely associated with resistance to bortezomib and dismal prognosis.2 Although the inhibitory effect of bortezomib is well recognized, its downstream mechanisms of cytotoxicity remain largely elusive and at times controversial.
The discovery of microRNA (miR) has revealed a new level of regulation of cell signaling and homeostasis.3 Deregulation of miR expression and miR-processing enzymes are described for several types of cancer. Dicer1, as one of the main components of the miR processing ensemble, has been implicated in the context of cancer initiation and evolution, especially in myeloma cells.4 The induction of miR-128 by mutant (Mut) p53 has also been observed in tumor cells, suggesting its role in the generation of chemoresistance.5
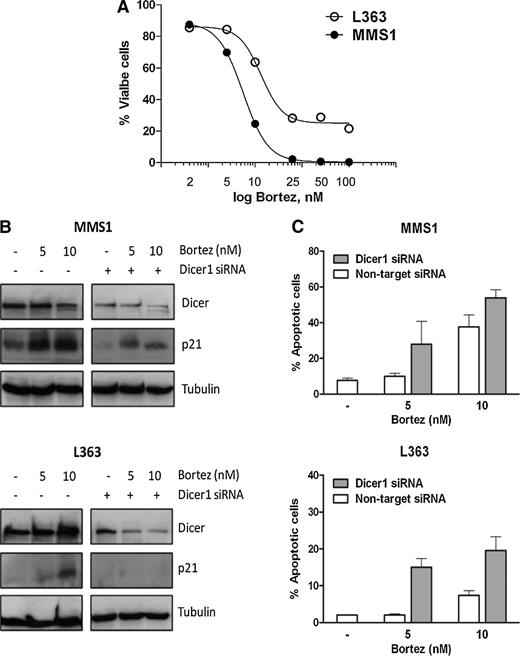
Here, the human myeloma cancer cell lines p53-Mut (L363) and p53 wild-type (p53-WT) (MMS1) were used as a model system to investigate the effects of bortezomib on MM cell growth. As shown in Figure 1A, we detected a dose-dependent decrease in the growth of both WT and p53-Mut myeloma cell lines with 50% inhibitory concentration values of 11.6 nM (against L363) and 7.4 nM (against MMS1) 24 hours after exposure to bortezomib. Having obtained this information, we next asked whether bortezomib modulates Dicer1 expression levels. As depicted in Figure 1B, we found bortezomib to decrease Dicer1 protein levels in MMS1 cells. In contrast, no change in Dicer1 expression levels was observed in p53-Mut L363 cells.
Effects of Dicer1 silencing on bortezomib-induced apoptosis in MM cells. (A) Representative dose-response curve of MMS1 and L363 cells after incubation for 24 hours with various bortezomib concentrations. Cell viability (%) was measured by annexin V/propidium iodide staining (AnnV/PI) (Invitrogen, Paisley, United Kingdom), and viable cells are defined as AnnV–/PI–. Results are shown as the mean of duplicates (n = 2). (B) Lysates were generated from Dicer1 knockdown with 100 nM short interfering RNA (siRNA) or nontarget siRNA control MMS1 and L363 cells treated with 5 to 10 nM bortezomib (Cell Signaling Technology, Danvers, MA) or vehicle for 24 hours, and the expression of Dicer1 (Cell Signaling Technology) and p21 (BD Transduction Laboratories, Heidelberg, Germany) proteins were analyzed by western blot. Tubulin served as a loading control. The image shows a representative of 3 independent experiments run on the same gel. Briefly, 4.5 × 105 MM cells were transiently transfected with Dicer1 siRNA (Cell Signaling Technology) or nontarget smart pool siRNA (Dharmacon, Lafayette, CO) using the TransIT-siQUEST transfection reagent (Mirus Bio, Madison, WI). MM cells were transfected with a solution containing 250 µL Opti-Mem medium (Invitrogen), 3.5 µL TransIT-siQUEST transfection reagent, and 100 nM Dicer1 siRNA or nontarget siRNA. Transfection efficiency was assessed by western blot. (C) Dicer1 knockdown increased apoptosis upon bortezomib treatment in MM cells. Dicer1 knockdown with 100 nM siRNA or nontarget siRNA control MMS1 and L363 cells was treated with 5 to 10 nM bortezomib or vehicle for 24 hours before an assessment of the percentage of AnnV+/PI+ cells by flow cytometry. Dicer1 knockdown potentiates the effect of bortezomib in L363 cells. The data presented are means ± SD of 3 experiments.
Effects of Dicer1 silencing on bortezomib-induced apoptosis in MM cells. (A) Representative dose-response curve of MMS1 and L363 cells after incubation for 24 hours with various bortezomib concentrations. Cell viability (%) was measured by annexin V/propidium iodide staining (AnnV/PI) (Invitrogen, Paisley, United Kingdom), and viable cells are defined as AnnV–/PI–. Results are shown as the mean of duplicates (n = 2). (B) Lysates were generated from Dicer1 knockdown with 100 nM short interfering RNA (siRNA) or nontarget siRNA control MMS1 and L363 cells treated with 5 to 10 nM bortezomib (Cell Signaling Technology, Danvers, MA) or vehicle for 24 hours, and the expression of Dicer1 (Cell Signaling Technology) and p21 (BD Transduction Laboratories, Heidelberg, Germany) proteins were analyzed by western blot. Tubulin served as a loading control. The image shows a representative of 3 independent experiments run on the same gel. Briefly, 4.5 × 105 MM cells were transiently transfected with Dicer1 siRNA (Cell Signaling Technology) or nontarget smart pool siRNA (Dharmacon, Lafayette, CO) using the TransIT-siQUEST transfection reagent (Mirus Bio, Madison, WI). MM cells were transfected with a solution containing 250 µL Opti-Mem medium (Invitrogen), 3.5 µL TransIT-siQUEST transfection reagent, and 100 nM Dicer1 siRNA or nontarget siRNA. Transfection efficiency was assessed by western blot. (C) Dicer1 knockdown increased apoptosis upon bortezomib treatment in MM cells. Dicer1 knockdown with 100 nM siRNA or nontarget siRNA control MMS1 and L363 cells was treated with 5 to 10 nM bortezomib or vehicle for 24 hours before an assessment of the percentage of AnnV+/PI+ cells by flow cytometry. Dicer1 knockdown potentiates the effect of bortezomib in L363 cells. The data presented are means ± SD of 3 experiments.
In MM cells, the cell cycle inhibitor p21WAF1 is reduced by increased proteasome activity, which fosters progression through the cell cycle. In addition, miR-106a, miR-106b, miR-17-5p, and miR-20b have been shown to critically participate in the regulation of cell cycle progression by downregulating p21WAF1.6-8 We next evaluated the p21 expression pattern that is associated with Dicer1 in response to bortezomib. Dicer1 reduction was found to inversely correlate with p21 protein accumulation in p53-WT MMS1 cells (Figure 1B). Notably, p53-Mut L363 cells failed to accumulate p21WAF1 protein. p21WAF1 stabilization was detected only with bortezomib at higher concentrations in a p53-independent manner in the p53-Mut cell line.
Consistent with these findings, bortezomib effectively inhibited cell growth and induced cell death in MMS1 cells with p53-WT but was less effective in inhibiting cell growth and inducing cell death for L363 cells with p53-Mut (Figure 1C). This indicates that p53 status–associated Dicer1 expression is an essential parameter for predicting bortezomib sensitivity. The data presented here illustrate that cells with p53-WT show a low level of Dicer1 and are sensitive to bortezomib, and myeloma cells with p53-Mut show a constant high level of Dicer1 and are less sensitive to bortezomib-mediated apoptosis induction.
Suzuki et al9 reported a role for p53 in promoting the maturation of miRs via interaction with the miR processing complex, DDR8-Drosha. Both p53-WT and p53-Mut have prominent but opposing roles at various levels in the process of miR maturation. Impaired processing of miR was associated with MM disease progression. The findings of Zhou et al8 clearly suggest that depletion of AGO2 or Dicer1, 2 key regulators for miR maturation and functionality, significantly decreased viability of myeloma cells. Knockdown of Dicer1 by siRNA increased sensitivity to bortezomib in p53-WT MMS1 cells. When Dicer1 expression was antagonized in the p53-Mut L363 cell line, a greater cytotoxic response was seen in treatment with bortezomib (Figure 1B-C). In conclusion, this study identified that Dicer1 knockdown can be exploited to sensitize myeloma for chemotherapy, even in p53-Mut cells.
Authorship
Acknowledgments: This work was supported by the Deutsche Jose Carreras Leukämie Stiftung (DJCLS R07/10) (G.S.).
Contribution: T.S.N. performed the laboratory work for this study and wrote the paper; G.S. made a contribution to conception and design.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Tatyana S. Nekova, Department of Internal Medicine II, Julius-Maximilian’s University of Wuerzburg, Josef-Schneider Strasse 2, 97080 Wuerzburg, Germany; e-mail: nekova_t@ukw.de.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal