Abstract
Erdheim-Chester disease (ECD) is a rare, non-Langerhans histiocytosis. Recent findings suggest that ECD is a clonal disorder, marked by recurrent BRAFV600E mutations in >50% of patients, in which chronic uncontrolled inflammation is an important mediator of disease pathogenesis. Although ∼500 to 550 cases have been described in the literature to date, increased physician awareness has driven a dramatic increase in ECD diagnoses over the last decade. ECD frequently involves multiple organ systems and has historically lacked effective therapies. Given the protean clinical manifestations and the lack of a consensus-derived approach for the management of ECD, we provide here the first multidisciplinary consensus guidelines for the clinical management of ECD. These recommendations were outlined at the First International Medical Symposium for ECD, comprised of a comprehensive group of international academicians with expertise in the pathophysiology and therapy of ECD. Detailed recommendations on the initial clinical, laboratory, and radiographic assessment of ECD patients are presented in addition to treatment recommendations based on critical appraisal of the literature and clinical experience. These formalized consensus descriptions will hopefully facilitate ongoing and future research efforts in this disorder.
Introduction
Erdheim-Chester disease (ECD) is a rare, non-Langerhans histiocytosis described by Jakob Erdheim and William Chester in 1930.1 Although 500 to 550 cases have been reported, the number has dramatically increased in the last 10 years due to increased recognition of the disease. Historically, ECD has been considered a variably aggressive histiocytic disorder of unclear origin with poor response to therapy. However, recent identification of the clonal nature of the disorder with at least one therapeutically relevant recurrent oncogenic somatic mutation has reformulated our understanding of the pathogenesis and clinical management of the disease.2 Currently, there are no universally accepted guidelines for the diagnosis and treatment of ECD and patients present to a variety of medical practitioners. Given this clinical diversity, there is a need to formalize recommendations for this disorder.
Methods
A comprehensive and multidisciplinary group of scientists and physicians engaged in the investigation and treatment of ECD convened to establish consensus recommendations for ECD diagnosis and therapy. This meeting occurred at the First International Medical Symposium for ECD on 31 October, 2013, organized by the ECD Global Alliance, an organization devoted to this disorder. A listing of ECD experts and referral centers is located on the Web sites of the ECD Global Alliance (http://www.erdheim-chester.org/) and Euro-Histio-Net (http://www.eurohistio.net/index_eng.html). The National Human Genome Research Institute of the National Institutes of Health is also conducting a natural history study of ECD patients; this study provides comprehensive evaluation including radiographic staging, detailed history, and physical examination.
Recommendations are formulated based on the level of evidence and agreement between experts as described in Table 1 and as performed in guidelines published for adults with Langerhans cell histiocytosis (LCH).3 Our recommendations specifically refer to adults, although the limited evidence about pediatric ECD is summarized separately. These guidelines present the unified experiences in the management of ECD by academic clinicians whose experience extends beyond current literature (supplemental Table 2).
Grades of recommendation
| Level of evidence . | Level of agreement between experts . |
|---|---|
| (A) Meta-analyses, high-quality systematic reviews, or randomized controlled trials | (2) 100% agreement between all experts |
| (B) Systematic reviews of case control or cohort studies | (1) ≥80% agreement between all experts |
| (C) Nonanalytic studies such as case reports, case series, and small retrospective studies | (0) Divergence of opinion (<80% agreement between all experts) |
| (D) Expert opinion |
| Level of evidence . | Level of agreement between experts . |
|---|---|
| (A) Meta-analyses, high-quality systematic reviews, or randomized controlled trials | (2) 100% agreement between all experts |
| (B) Systematic reviews of case control or cohort studies | (1) ≥80% agreement between all experts |
| (C) Nonanalytic studies such as case reports, case series, and small retrospective studies | (0) Divergence of opinion (<80% agreement between all experts) |
| (D) Expert opinion |
Diagnosis and clinical features
Pathogenesis.
There has been longstanding uncertainty about the underlying etiology of ECD, and it has been considered to be a nonneoplastic inflammatory disorder as well as a clonal neoplastic disorder.4-6 Recent discovery of BRAFV600E mutations in LCH by Badalian-Very et al7 yielded the first identification of a bona fide oncogenic alteration in this disorder. Estimates of BRAFV600E mutation frequencies in ECD currently range between 38% and 68% in most reports, with one recent report suggesting that nearly 100% (18/18) ECD patients have the mutation if sufficiently sensitive techniques are used.7-11 In addition, an oncogenic NRASQ61R mutation was identified in an ECD patient, further highlighting the importance of mitogen-activated protein kinase signaling to ECD pathogenesis.12
In parallel to these observations supporting the clonal nature of ECD, ECD histiocytes have been found to express a pattern of proinflammatory cytokines and chemokines responsible for local activation and recruitment of histiocytes.13 Also, analyses of the sera of ECD patients have identified a unique inflammatory cytokine signature characteristic of ECD.14 This consists of elevated levels of interferon (IFN)-α, interleukin (IL)-12, monocyte chemotactic protein-1, and decreased IL-4 and IL-7 in ECD patients relative to controls. Based on these studies, ECD can now be defined as a clonal disorder marked by frequent hyperactivation of mitogen-activated protein kinase signaling in which an inflammatory milieu is important in the pathogenesis and clinical manifestations of the disease.
Important questions regarding the cell of origin of ECD, the somatic genetic alterations present in ECD patients without BRAFV600E mutations, and the proximate cause of immune dysregulation in ECD have yet to be fully answered. Furthermore, the relationship between ECD and LCH is yet to be understood, as syndromes of ECD-LCH overlap have been clearly documented and occur in as many as 12% of ECD cases.15-19
Epidemiology.
The majority of ECD patients are diagnosed between the ages of 40 and 70 years. The largest investigation of the epidemiology of ECD comes from a series of 53 patients evaluated at Pitie-Salpêtrière Hospital, where the mean age of diagnosis was 55 years with a range of 60 to 80 years.20 Based on this series, ECD appears to have a male predominance of 73%.20
Diagnostic criteria.
The diagnosis of ECD is made by identifying distinctive histopathological findings in the appropriate clinical and radiologic context. Lesional tissue demonstrates infiltration of typically foamy or lipid-laden histiocytes with admixed or surrounding fibrosis (Figure 1E). Touton giant cells are often present. On immunohistochemical (IHC) staining, ECD histiocytes are positive for CD68 (Figure 1F), CD163, and Factor XIIIa, and negative for CD1a and Langerin (CD207). Positivity for S100 has been observed rarely. This differentiates ECD from LCH, where Langerhans cells are positive for CD1a, S100, and Langerin (Table 2). ECD histiocytes are morphologically and immunohistochemically identical to those in juvenile xanthogranuloma (JXG), and it has been posited that ECD is a variant JXG with predominantly noncutaneous involvement.21 Table 2 describes the shared and differentiating pathological, clinical, and radiographic features among systemic histiocytic disorders affecting adults.
Characteristic histopathologic and radiographic findings of ECD. (A) PET and (B) 99mTc imaging demonstrating symmetric diametaphyseal radiotracer uptake in the long bones of the legs (arrows) commonly seen in ECD patients. R indicates the patient's right side. (C) CT and (D) MRI scans revealing sclerotic lesions of the metaphyses of femur and tibia (arrows). (E) Hematoxylin-eosin–stained biopsy section of ECD lesion revealing lipid-laden histiocytes characteristic of ECD. (F) IHC stain for CD68 revealing positivity of histiocytes.
Characteristic histopathologic and radiographic findings of ECD. (A) PET and (B) 99mTc imaging demonstrating symmetric diametaphyseal radiotracer uptake in the long bones of the legs (arrows) commonly seen in ECD patients. R indicates the patient's right side. (C) CT and (D) MRI scans revealing sclerotic lesions of the metaphyses of femur and tibia (arrows). (E) Hematoxylin-eosin–stained biopsy section of ECD lesion revealing lipid-laden histiocytes characteristic of ECD. (F) IHC stain for CD68 revealing positivity of histiocytes.
Systemic histiocytic disorders of adults: ECD, LCH, and RDD
| . | ECD . | LCH . | RDD . |
|---|---|---|---|
| Histopathologic features | |||
| CD68 | + | + | + |
| CD163 | + | + | + |
| CD1a | − | + | − |
| CD207 | − | + | − |
| S100 | − or weakly + | + | + |
| Factor XIIIa | + | − | − |
| Touton giant cells | + | − | − |
| Other characteristic features of lesional histiocytes | Xanthomatous features | Birbeck granules on electron microscopy | Intracytoplasmic lymphocytes (emperipolesis) |
| Fibrosis | |||
| Organ system involvement | |||
| Skin24,95 | Xanthelasma | Scaly erythematous patches | Firm, indurated papules |
| Yellow or red-brown plaques | |||
| Heart15 | Pericardial effusion | Reported, but uncharacteristic | Reported, but uncharacteristic |
| Myocardial infiltration, right atrial mass | |||
| Periaortic sheathing (“coated aorta”) | |||
| Lungs43,96 | Interlobular septal thickening, ground-glass or centrilobular opacities on CT | Nodular and cystic changes in upper and middle lobes | Reported, but uncharacteristic |
| Retroperitoneum | Perinephric infiltration | Reported, but uncharacteristic | Reported, but uncharacteristic |
| Liver and spleen | Rare | Uncommon but constitutes high-risk disease | Reported, but uncharacteristic |
| Bone33 | Femurs and tibia | Craniofacial bones, proximal limbs, pelvis, scapula | Reported, but uncharacteristic |
| Bone pain | |||
| Lymph nodes | Reported, but uncharacteristic | Uncommon but constitutes high-risk disease | Cervical LN, axillary, inguinal, paraaortic, or mediastinal |
| CNS48,97,98 | Cerebellar or brain stem lesions | Cerebellar or brain stem lesions | Dural-based lesions |
| Dural-based lesions | Dural-based lesions | ||
| Brain parenchymal lesions | Brain parenchymal lesions | ||
| Noninfiltrative neuro-degeneration |
| . | ECD . | LCH . | RDD . |
|---|---|---|---|
| Histopathologic features | |||
| CD68 | + | + | + |
| CD163 | + | + | + |
| CD1a | − | + | − |
| CD207 | − | + | − |
| S100 | − or weakly + | + | + |
| Factor XIIIa | + | − | − |
| Touton giant cells | + | − | − |
| Other characteristic features of lesional histiocytes | Xanthomatous features | Birbeck granules on electron microscopy | Intracytoplasmic lymphocytes (emperipolesis) |
| Fibrosis | |||
| Organ system involvement | |||
| Skin24,95 | Xanthelasma | Scaly erythematous patches | Firm, indurated papules |
| Yellow or red-brown plaques | |||
| Heart15 | Pericardial effusion | Reported, but uncharacteristic | Reported, but uncharacteristic |
| Myocardial infiltration, right atrial mass | |||
| Periaortic sheathing (“coated aorta”) | |||
| Lungs43,96 | Interlobular septal thickening, ground-glass or centrilobular opacities on CT | Nodular and cystic changes in upper and middle lobes | Reported, but uncharacteristic |
| Retroperitoneum | Perinephric infiltration | Reported, but uncharacteristic | Reported, but uncharacteristic |
| Liver and spleen | Rare | Uncommon but constitutes high-risk disease | Reported, but uncharacteristic |
| Bone33 | Femurs and tibia | Craniofacial bones, proximal limbs, pelvis, scapula | Reported, but uncharacteristic |
| Bone pain | |||
| Lymph nodes | Reported, but uncharacteristic | Uncommon but constitutes high-risk disease | Cervical LN, axillary, inguinal, paraaortic, or mediastinal |
| CNS48,97,98 | Cerebellar or brain stem lesions | Cerebellar or brain stem lesions | Dural-based lesions |
| Dural-based lesions | Dural-based lesions | ||
| Brain parenchymal lesions | Brain parenchymal lesions | ||
| Noninfiltrative neuro-degeneration |
Highly disease-specific findings are presented in bold.
In addition to histological features, the radiographic finding of symmetric diaphyseal and metaphyseal osteosclerosis in the legs is nearly always present in ECD (Figure 1A-D). This is best visualized by radiotracer uptake in the distal ends of the femurs and the proximal and distal tibia by bone scan, and less sensitively by positron emission tomography (PET). Bone lesions may be visualized on computed tomography (CT) or magnetic resonance imaging (MRI) but are often missed on plain films.22,23 Approximately 4% of ECD patients lack radiographic findings of osteosclerosis of the femurs.15 In these cases, the diagnosis of ECD is based on histopathology and other classic organ involvement. Second, dense infiltration of perinephric fat, described as a “hairy kidney” based on its appearance on CT, is a highly prevalent (68% of cases) and iconic radiographic finding (Figure 2A).22 Even in circumstances with highly suggestive clinical and imaging features, biopsy is necessary to confirm the diagnosis and establish BRAF mutational status (Grade 2C).
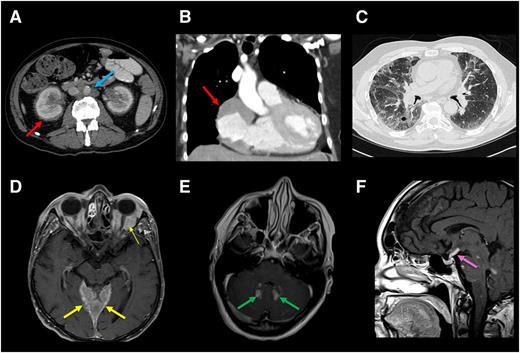
Radiographic findings of internal organ systems commonly affected by ECD. (A) Axial CT scan of abdomen of an ECD patient demonstrating dense infiltration of perinephric fat commonly seen in ECD and referred to as a “hairy kidney” appearance (red arrow). Circumferential soft-tissue sheathing of the thoracic aorta seen in a subset of ECD patients and referred to as a “coated aorta” (blue arrow). Right atrial mass is demonstrated in B. (C) Lung parenchymal infiltration on chest CT in an ECD patient. Axial postgadolinium T1 MRI in (D) demonstrates expansile enhancement of the pachymeninges (thick arrow) as well as orbital masses (thin arrow) and (E) enhancing lesions in the dentate nuclei of the cerebellum. (F) Sagittal postgadolinium T1 MRI shows thickening and enhancement of the pituitary stalk.
Radiographic findings of internal organ systems commonly affected by ECD. (A) Axial CT scan of abdomen of an ECD patient demonstrating dense infiltration of perinephric fat commonly seen in ECD and referred to as a “hairy kidney” appearance (red arrow). Circumferential soft-tissue sheathing of the thoracic aorta seen in a subset of ECD patients and referred to as a “coated aorta” (blue arrow). Right atrial mass is demonstrated in B. (C) Lung parenchymal infiltration on chest CT in an ECD patient. Axial postgadolinium T1 MRI in (D) demonstrates expansile enhancement of the pachymeninges (thick arrow) as well as orbital masses (thin arrow) and (E) enhancing lesions in the dentate nuclei of the cerebellum. (F) Sagittal postgadolinium T1 MRI shows thickening and enhancement of the pituitary stalk.
Clinical and radiographic features.
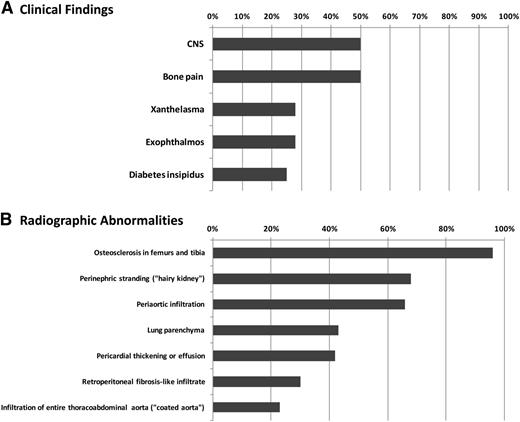
Figure 3 presents the estimated frequencies of clinical and radiographic findings in ECD patients based on the largest single-center experience to date.15 Delayed or erroneous diagnosis is common, and from symptom-onset to diagnosis can span from months to decades. As mentioned, ECD shares clinical features with LCH (Table 2) but bears little clinical similarity to other non-LCH histiocytoses, with the exception of sinus histiocytosis with massive lymphadenopathy (Rosai-Dorfman disease).24,25 Although infiltration of nearly every organ system has been reported in ECD,26-31 the tissues most commonly affected include the skeleton, retroperitoneum, and orbit in addition to the cardiovascular, pulmonary, neurologic, and endocrine systems (Figure 3). Below is a discussion of the organ systems affected by ECD with a consideration of the differential diagnosis for each regional presentation.
Frequencies of recurrent clinicoradiographic findings in ECD patients. Frequency of recurrent clinical findings (A) and radiographic findings (B) in ECD based on a multicenter observational cohort study between 1981 and 2010.20 Frequency of patients with reported finding is listed on the x-axis.
Frequencies of recurrent clinicoradiographic findings in ECD patients. Frequency of recurrent clinical findings (A) and radiographic findings (B) in ECD based on a multicenter observational cohort study between 1981 and 2010.20 Frequency of patients with reported finding is listed on the x-axis.
Osseous manifestations.
Despite the near universality of long bone involvement in ECD, only 50% of patients describe bone pain.32 The distribution of sclerotic lesions in ECD differs from that of LCH in that the latter commonly involves the calvarium, facial bones, proximal limbs, pelvis, and scapula rather than the distal limbs as in ECD.33
Cardiovascular manifestations.
Cardiovascular involvement is common but frequently asymptomatic and detected incidentally by CT or MRI.34,35 The most common abnormality is circumferential soft-tissue sheathing of the thoracic and abdominal aorta and its branches visualized on CT scan, the so-called “coated aorta,” present in up to two-thirds of patients (Figure 2A).15,36 If renal arteries are involved, renovascular hypertension may develop and ultimately require stenting. Coronary arterial disease resulting in myocardial infarction has been described.36-38 Pericardial disease occurs in 40% to 45% of patients and can present with pericarditis, effusion, and even tamponade.32,39,40 Mural pseudo-tumoral infiltration of the right atrium is present in up to one-third of patients, visualized clearly on MRI as a mass lesion (Figure 2B), rarely causing valvular dysfunction and conduction abnormalities.34,35 Diffuse infiltration of the myocardium or interatrial septum has been described, occasionally leading to heart failure.41,42 Involvement of intrahepatic veins causing portal hypertension as well as the mesenteric vessels leading to ischemia have been seen but not published.
Pulmonary manifestations.
Radiographic lung involvement may be present in up to one-half of cases, involving either the lung parenchyma or the pleura (Figure 2C).15,43 Plain films are typically normal, but high-resolution CT may demonstrate interlobular septal thickening, ground-glass opacities, or centrilobular opacities.22 Frank consolidations are uncommon. Fluid from bronchoalveolar lavage, if performed, may contain macrophages and foamy histiocytes. Pulmonary involvement is often asymptomatic but can rarely manifest as cough or dyspnea. Spirometry may demonstrate restrictive features and decreased diffusion capacity.
Central nervous system, orbital, and neuroendocrine manifestations.
The frequency of central nervous system (CNS) involvement in ECD varies from 25% to 50%.22,44 Parenchymal CNS lesions are a significant cause of functional disability in ECD and have been found in one series to be an independent predictor of death.45 Infiltrations can occur throughout the neuraxis, both in the intra-axial and extra-axial compartments. Expansile, gadolinium-enhancing lesions of the pachymeninges can occur overlying the cerebral hemispheres or in the cerebellar tentorium (Figure 2D). They can appear similar to meningiomas, granulomatous diseases, or meningeal infiltration by Rosai-Dorfman disease or LCH.32,33 Lesions present with focal symptoms referable to compression of local structures or, when disease is diffuse and bulky, with generalized deterioration of cognition and gait. Intra-axially, ECD manifests more commonly in the dentate nuclei of the cerebellum or in the pons, causing progressive cerebellar symptoms such as ataxia and dysarthria as well as brainstem symptoms (Figure 2E).22,25,46 These abnormalities tend to be gadolinium-enhancing and may be mistaken for primary or metastatic tumors, demyelinating disease, inflammatory processes, or leukodystrophies.28,29 Similar intracranial lesions have been reported in JXG.47 Infiltrative CNS lesions in LCH have similar distribution and radiographic appearances to lesions in ECD, with the exception of meningeal lesions, which are more expansile in ECD, and the spinal cord, which is spared in LCH. A degenerative atrophic process in the posterior fossa is an uncommon but well-described manifestation of LCH48 ; neurodegenerative phenomena in ECD remain uncharacterized.
Unilateral or bilateral infiltration of the orbits occurs in ∼25% of patients and can present as exophthalmos, retro-orbital pain, oculomotor palsies, or blindness.15,49 These pseudotumoral lesions have a differential diagnosis that includes Graves disease, granulomatous disease, lymphoma, and giant cell arteritis.
Diabetes insipidus is a shared feature of ECD and LCH, occurring in ∼25% of ECD patients, although several endocrinopathies have been reported, including hyperprolactinemia, gonadotropin insufficiency, and hypotestosteronism.15 Radiographically, the pituitary gland, stalk, and hypothalamus may be normal; alternatively, enlargement and abnormal enhancement of these structures can be appreciated on MRI with or without accompanying endocrinopathy (Figure 2F).50
Retroperitoneal manifestations.
Infiltration of perinephric tissues leading to the “hairy kidney” is common, as is hydronephrosis and ureteral narrowing (Figure 2A). Furthermore, ∼30% ECD patients present with imaging features suggestive of retroperitoneal fibrosis; however, unlike idiopathic retroperitoneal fibrosis, the pelvic ureters and inferior vena cavae are typically spared.15 Ureteral stenting or even nephrostomy may be required if hydronephrosis is associated with renal insufficiency.
Cutaneous manifestations
The most common cutaneous lesion of ECD is xanthelasma, occurring as yellow eyelid plaques in approximately one-third of patients.15 Other sites are the face, neck, axilla, trunk, and groin and may appear as yellow or red-brown papules that merge into plaques. It is impossible to distinguish between ECD and adult JXG on the basis of skin lesions alone, but unlike ECD, JXG is less commonly a multi-system disease.25
Proposed categories of disease.
It has been reported that patients with CNS disease suffer adverse outcomes with interferon-based treatment compared with patients with other disease sites, supporting the notion that sites of involvement influence prognosis.20 One disease site may dominate the clinical presentation and require focused treatment in addition to treatment of underlying ECD. Rarely, some patients have indolent and asymptomatic disease that warrants expectant management rather than active treatment. As a result, we propose that ECD be categorized as asymptomatic and symptomatic (Table 3). Symptomatic ECD can be further categorized as CNS dominant, cardiac dominant, vascular dominant, endocrine dominant, retroperitoneal dominant, pulmonary dominant, and multisystem. Severe involvement of essentially any organ system constitutes “high-risk” disease; therefore, the clinical phenotype of each patient is best characterized by the most pathophysiogically affected organ. We believe that this categorization will enhance the description of this protean disease, facilitate appropriate treatment, and provide utility as new treatments are developed.
Proposed ECD classification
| Clinical severity and organ system dominance . |
|---|
| Asymptomatic or minimally symptomatic ECD |
| Cutaneous-dominant disease |
| Minimal or asymptomatic bone disease |
| Symptomatic ECD |
| CNS dominant |
| Cardiac dominant |
| Retroperitoneal dominant |
| Orbital-craniofacial dominant |
| Neuroendocrine dominant |
| Pulmonary dominant |
| Multisystem |
| Clinical severity and organ system dominance . |
|---|
| Asymptomatic or minimally symptomatic ECD |
| Cutaneous-dominant disease |
| Minimal or asymptomatic bone disease |
| Symptomatic ECD |
| CNS dominant |
| Cardiac dominant |
| Retroperitoneal dominant |
| Orbital-craniofacial dominant |
| Neuroendocrine dominant |
| Pulmonary dominant |
| Multisystem |
Baseline evaluation and BRAF V600E mutation assessment
Baseline evaluation.
Table 4 presents the recommended initial assessment of patients. CT scan of chest, abdomen, and pelvis, (18F)-fluorodeoxyglucose (FDG) PET of the entire body including the brain and distal extremities, MRI of the brain with gadolinium, and cardiac MRI are recommended in all patients (Grade C2). Additional studies may be appropriate to evaluate clinical or laboratory findings or to delineate imaging abnormalities in a detailed manner (Grade C1). Although 99mTc bone scans may aid in the diagnosis of ECD by demonstrating the iconic lesions, the sensitivity of PET scans for extra-osseous involvement has rendered FDG-PET the nuclear medicine study of choice for evaluation of overall ECD burden.51-53 Laboratory studies are performed to assess for renal insufficiency, cytopenias, markers of inflammation, and evidence of endocrinopathy (Grade C1). The goal of the comprehensive baseline assessment is to characterize the burden of disease and detect abnormalities that may emerge clinically in the future.
Baseline clinical evaluation recommendations for patients diagnosed with ECD
| Medical history . | Radiological evaluation . | Physical examination . | Laboratory evaluation . |
|---|---|---|---|
| Constitutional: fevers, night sweats, fatigue HEENT: double vision, retro-orbital pain Cardiovascular: dyspnea, orthopnea Pulmonary: dyspnea, cough Musculoskeletal: bone pain Dermatologic: xanthelasma, rash Endocrine: polydipsia/polyuria, gynecomastia, decreased libido Neurologic: ataxia, dysarthria, dysphagia, cognitive decline Psychiatric: depression, disinhibition, inappropriate laughing or crying | All patients CT chest, abdomen, and pelvis PET/CT including distal extremities MRI brain with contrast and detailed examination of the sella turcica Cardiac MRI Selected patients based on symptoms or organ involvement MRI orbit with contrast Renal artery ultrasound High-resolution CT chest Pulmonary function tests Testicular ultrasound Electromyography | HEENT: xanthelasma, exophthalmos Cardiac: hypertension, irregular pulse, cardiomegaly, murmurs, ECG abnormalities Pulmonary: diminished aeration, rales Neurologic: disconjugate gaze, cranial nerve palsies, dysarthria, ataxic or magnetic gait, hyperreflexia Psychiatric: pseudobulbar affect | Complete blood count with differential Comprehensive metabolic panel Erythrocyte sedimentation rate C-reactive protein Morning urine osmolality Morning serum cortisol TSH and free T4 Prolactin, testosterone (males), LH, FSH Vitamin B12, thiamine levels BRAF V600 genotyping |
| Medical history . | Radiological evaluation . | Physical examination . | Laboratory evaluation . |
|---|---|---|---|
| Constitutional: fevers, night sweats, fatigue HEENT: double vision, retro-orbital pain Cardiovascular: dyspnea, orthopnea Pulmonary: dyspnea, cough Musculoskeletal: bone pain Dermatologic: xanthelasma, rash Endocrine: polydipsia/polyuria, gynecomastia, decreased libido Neurologic: ataxia, dysarthria, dysphagia, cognitive decline Psychiatric: depression, disinhibition, inappropriate laughing or crying | All patients CT chest, abdomen, and pelvis PET/CT including distal extremities MRI brain with contrast and detailed examination of the sella turcica Cardiac MRI Selected patients based on symptoms or organ involvement MRI orbit with contrast Renal artery ultrasound High-resolution CT chest Pulmonary function tests Testicular ultrasound Electromyography | HEENT: xanthelasma, exophthalmos Cardiac: hypertension, irregular pulse, cardiomegaly, murmurs, ECG abnormalities Pulmonary: diminished aeration, rales Neurologic: disconjugate gaze, cranial nerve palsies, dysarthria, ataxic or magnetic gait, hyperreflexia Psychiatric: pseudobulbar affect | Complete blood count with differential Comprehensive metabolic panel Erythrocyte sedimentation rate C-reactive protein Morning urine osmolality Morning serum cortisol TSH and free T4 Prolactin, testosterone (males), LH, FSH Vitamin B12, thiamine levels BRAF V600 genotyping |
Acquisition of lesional tissue.
Selection of biopsy site and histopathologic confirmation of ECD is challenging. Shave biopsy of cutaneous lesions, such as xanthelasma, is the least invasive procedure and often yields suitable diagnostic tissue. Regardless of biopsy site, efforts should be made to obtain several samples, because the yield of a single biopsy may be low as histologic changes vary from field to field. In addition, bone samples must be decalcified for histopathologic interpretation, rendering the material unsuitable for genetic analysis; therefore, obtaining an ample number of cores and preserving fresh material without decalcification for purposes of DNA extraction are critical.
The classic histopathologic findings in ECD have been discussed; however, it must be noted that lesional tissue often does not demonstrate the classic foamy histiocytic infiltrate. A variety of findings are often encountered, including nonspecific inflammation with admixed fibrosis or even fibrosis alone with scant histiocytes (Figure 4).
Heterogeneous histopathologic findings in ECD lesional tissue. Brain biopsy in ECD yielded mostly lamellar fibrotic tissue (A) with only small regions (B) with lipid-laden histiocytes (yellow arrows) and Touton giant cells (blue arrow). (C) Another sample of a CNS lesion demonstrated a florid lymphohistiocytic infiltrate with regions of pale, nonfoamy histiocytes (inset). (D) Reactive astrocytes and Rosenthal fibers were seen in a separate CNS biopsy from the same case. (E) Biopsy of perinephric infiltrate on low power view demonstrates perirenal fat with septa widened by fibrous tissue and scant inflammatory infiltrate. The stranding apparent at low power correlates with the known radiographic appearance. (F) Higher power in this case shows a Touton giant cell but few histiocytes. (G) Pericardial biopsy shows pale and granular histiocytes without foamy appearance. Occasional lymphocytes, plasma cells, and eosinophils are present. (H) Lung biopsy from the same patient taken concurrently demonstrates septal fibrosis with scant cellular infiltrate including histiocytes. Higher power (inset) shows pale (nonfoamy) histiocytes.
Heterogeneous histopathologic findings in ECD lesional tissue. Brain biopsy in ECD yielded mostly lamellar fibrotic tissue (A) with only small regions (B) with lipid-laden histiocytes (yellow arrows) and Touton giant cells (blue arrow). (C) Another sample of a CNS lesion demonstrated a florid lymphohistiocytic infiltrate with regions of pale, nonfoamy histiocytes (inset). (D) Reactive astrocytes and Rosenthal fibers were seen in a separate CNS biopsy from the same case. (E) Biopsy of perinephric infiltrate on low power view demonstrates perirenal fat with septa widened by fibrous tissue and scant inflammatory infiltrate. The stranding apparent at low power correlates with the known radiographic appearance. (F) Higher power in this case shows a Touton giant cell but few histiocytes. (G) Pericardial biopsy shows pale and granular histiocytes without foamy appearance. Occasional lymphocytes, plasma cells, and eosinophils are present. (H) Lung biopsy from the same patient taken concurrently demonstrates septal fibrosis with scant cellular infiltrate including histiocytes. Higher power (inset) shows pale (nonfoamy) histiocytes.
BRAF V600E mutational testing.
Given the potential therapeutic implications of BRAFV600E mutations, accurate detection of the mutation is important (Grade C2). The highly variable histiocyte content present in many ECD biopsy samples influences BRAF mutational testing results.11 Moreover, the frequency of bone biopsies in ECD presents additional challenge to identifying of BRAF mutations. For these reasons, it is strongly encouraged to confirm negative BRAF V600E testing in ECD using >1 genotyping modality and/or genotyping from >1 anatomic site (particularly when bone lesions are found to be BRAF wild-type) (Grade C1).
Numerous molecular tests to determine BRAF genotype exist (recently reviewed54 ). In addition to molecular tests, IHC analysis of paraffin sections using the BRAFV600E mutant specific antibody (VE1) is possible. In several comparisons of BRAF mutational testing using IHC vs high-resolution melt curve and Sanger sequencing analysis in paraffin-embedded melanoma specimens, IHC had sensitivity and specificity >95%.55,56 Whenever possible, VE1+ IHC staining should be confirmed with molecular testing (Grade D1).
Given the difficulties associated with accessing lesions and heterogeneity of biopsy specimens in ECD, it is important to note commercial options for BRAFV600E mutational testing using circulating cell-free DNA.57 Further testing of cell-free DNA compared with genotyping of tissue-derived DNA in ECD is under investigation.
Treatment
There have been few prospective therapeutic studies and no randomized controlled trials in ECD. In general, initiation of therapy is recommended for all patients rather than observation, with the uncommon exception of patients with asymptomatic disease (Grade D1). The evidence for various therapeutic and supportive strategies is summarized (Table 5; supplemental Table 1). Given the paucity of prospective studies and limited data to support efficacious therapies, treatment in the setting of a clinical trial is recommended for all patients whenever available.
Treatment recommendations for ECD patients
| Therapy . | Treatment . | Dose and schedule . | Level of recommendation* . | Comment . |
|---|---|---|---|---|
| Clinical trial (first or second-line treatment) | Vemurafenib (NCT01524978) | 480-960 mg PO twice daily† | Grade C2 | Dramatic efficacy described in 3 cases81 and anecdotal experience with off-label use are known. Enrollment in prospective clinical trial is essential to document efficacy and potential toxicities as well as determine duration of therapy. |
| Tocilizumab (NCT01727206) | Per clinical trial guideline | Grade C2 | Phase II clinical trial of this anti-IL6 antibody in ECD is underway based on rationale of systemic elevation of IL-6 documented in ECD patients.14 | |
| Sirolimus and prednisone (ACTRN12613001321730) | Per clinical trial guideline | Grade C2 | Prospective trial underway based on concept of interfering with immune dysregulation in ECD. Moreover, recent identification of activating RAS mutation in ECD12 may provide rationale for mTOR inhibition by sirolimus in certain ECD patients. | |
| First-line | PEG-IFNα15 | 135 μg SC/wk (standard dose) or 180 μg SC/wk (high dose) | Grade C2 | Currently the therapy with largest clinical evidence-base in ECD.59-62 Case series have demonstrated survival benefit with the use of some form of IFN-α.45 High-dose IFN-α ended for patients with CNS or cardiac involvement.61 |
| IFN-α15 | 3 mIU SC TIW (standard dose) or 6-9 mIU SC TIW (high dose) | Grade C2 | Optimal duration of therapy unknown but use up to 3 y has been described.62 | |
| Anakinra65,98 | 100 mg SC daily | Grade C1 | A single case series describes efficacy of anakinra in treatment of constitutional symptoms in ECD.64-66 Appears to be less efficacious than IFN-α for therapy of patients with CNS or cardiac involvement. | |
| Second-line | Cladribine3,77 | 6 mg/m2 IV daily for 5 days every 4 wks | Grade C1 | Used frequently in clinical therapy of systemic LCH and ECD, but published reports of its efficacy are few. |
| Imatinib83,85,99 | 400 mg PO daily | Grade C0 | Results in 7 ECD patients treated with imatinib have been mixed, although it appears that it may be more effective in less advanced or severe forms of the disease.84,85 | |
| Infliximab68 | 5 mg/mg IV every 6 wks | Grade C1 | Four patients with cardiac disease refractory to treatment with IFN-α had clinical improvement when treated infliximab.68,100 |
| Therapy . | Treatment . | Dose and schedule . | Level of recommendation* . | Comment . |
|---|---|---|---|---|
| Clinical trial (first or second-line treatment) | Vemurafenib (NCT01524978) | 480-960 mg PO twice daily† | Grade C2 | Dramatic efficacy described in 3 cases81 and anecdotal experience with off-label use are known. Enrollment in prospective clinical trial is essential to document efficacy and potential toxicities as well as determine duration of therapy. |
| Tocilizumab (NCT01727206) | Per clinical trial guideline | Grade C2 | Phase II clinical trial of this anti-IL6 antibody in ECD is underway based on rationale of systemic elevation of IL-6 documented in ECD patients.14 | |
| Sirolimus and prednisone (ACTRN12613001321730) | Per clinical trial guideline | Grade C2 | Prospective trial underway based on concept of interfering with immune dysregulation in ECD. Moreover, recent identification of activating RAS mutation in ECD12 may provide rationale for mTOR inhibition by sirolimus in certain ECD patients. | |
| First-line | PEG-IFNα15 | 135 μg SC/wk (standard dose) or 180 μg SC/wk (high dose) | Grade C2 | Currently the therapy with largest clinical evidence-base in ECD.59-62 Case series have demonstrated survival benefit with the use of some form of IFN-α.45 High-dose IFN-α ended for patients with CNS or cardiac involvement.61 |
| IFN-α15 | 3 mIU SC TIW (standard dose) or 6-9 mIU SC TIW (high dose) | Grade C2 | Optimal duration of therapy unknown but use up to 3 y has been described.62 | |
| Anakinra65,98 | 100 mg SC daily | Grade C1 | A single case series describes efficacy of anakinra in treatment of constitutional symptoms in ECD.64-66 Appears to be less efficacious than IFN-α for therapy of patients with CNS or cardiac involvement. | |
| Second-line | Cladribine3,77 | 6 mg/m2 IV daily for 5 days every 4 wks | Grade C1 | Used frequently in clinical therapy of systemic LCH and ECD, but published reports of its efficacy are few. |
| Imatinib83,85,99 | 400 mg PO daily | Grade C0 | Results in 7 ECD patients treated with imatinib have been mixed, although it appears that it may be more effective in less advanced or severe forms of the disease.84,85 | |
| Infliximab68 | 5 mg/mg IV every 6 wks | Grade C1 | Four patients with cardiac disease refractory to treatment with IFN-α had clinical improvement when treated infliximab.68,100 |
PO, by mouth; SC, subcutaneous.
Grade and level of recommendation definitions are listed in Table 1.
960 mg twice daily is the FDA-approved dose of vemurafenib for melanoma; however, anecdotally, in most ECD patients, 480 mg twice daily is better tolerated and has been efficacious.
Interferon-α-2a and pegylated interferon-α.
Currently, the therapeutic modality with the largest amount of supporting evidence in ECD is IFN-α and pegylated IFN-α (PEG-IFN-α) (Grade C2). The efficacy of IFN-α in ECD was noted in 2005,58 and later the outcome of >60 ECD patients treated with some form of IFN-α has been described.59-62 In the largest single series, a prospective, nonrandomized, observational cohort study of 53 ECD patients, 46 patients treated with IFN-α or PEG-IFNα interferon significantly improved overall survival compared with other therapies and was an independent predictor of improved survival in multivariate analysis.45 Although the optimal dose of IFN-α/PEG-IFN-α is not established, 3 million units (mIU) 3 times/wk of IFN-α has been repeatedly shown to decrease lesional burden (Grade C1).58-60 In a case series of 8 patients with severe disease, including CNS and cardiac involvement, “standard” dose61 IFN-α was ineffective and high-dose IFN-α (IFN-α >18 mIU/wk or PEG-IFN-α>180 μg/wk) had greater efficacy. The optimal duration of IFN-α is likewise unclear in ECD, but “long-term” (up to 3 years) treatment with high-dose”IFN-α (9 mIU 3 times/wk [TIW]) or PEG-IFNa (180 µg/wk) was found in a study of 24 patients to have greater efficacy in high-risk ECD with stabilization or improvement in 64% of CNS disease and 79% of cardiac disease.62 There is no firmly established dose-equivalence between IFN-α and PEG-IFN-α, although PEG-IFN-α has been found to be more effective in sustaining response in the treatment of hepatitis C at 135 to 185 μg/wk compared with IFN-α 9 mIU/wk.63 IFN-α and PEG-IFN-α have several potential toxicities, including constitutional (fever, fatigue, flu-like symptoms, myalgias, and arthralgias), neuropsychiatric, and gastrointestinal symptoms, alopecia and pruritus, transaminitis, and myelosuppression. Side effects were not significantly different between dose levels of IFN-α in the above-mentioned series,62 although PEG-IFN-α is generally thought to be better tolerated. The consensus recommendation is in favor of PEG-IFN-α, with the dosage determined by the severity and organ dominance of disease.
Anticytokine directed therapy (anakinra, infliximab, tocilizumab).
Because IFN-α is thought to exert beneficial effects by suppressing the effect of IL-1, treatment with anakinra, a recombinant IL-1R antagonist, has been attempted and found effective in a small number of reported patients and many more unreported experiences. Reduction in disease burden as well as proinflammatory cytokines has been observed in 5 single patient case reports of treatment with anakinra at doses of 1 to 2 mg/kg/day.64-67 Treatment was well tolerated and particularly efficacious for bone pain and constitutional symptoms. Cardiac disease was successfully treated in one patient,67 although successful treatment of CNS ECD has not been reported with anakinra. Common side effects include injection site reactions, headache, arthralgias, and nasopharyngitis. Currently, there is less evidence to support anaknira’s use as first-line treatment than IFN-α, but it is reasonable therapy for many patients, especially those without CNS involvement and with prominent osseous or constitutional symptoms (Grade D1).
Additional anticytokine directed therapies have been investigated in ECD, including infliximab and tocilizumab. Four patients with cardiac disease refractory to IFN-α had clinical improvement and reduction in circulating cytokine levels when treated infliximab, an anti-tumor necrosis factor-α antibody. Although further study of this therapy is necessary, it may be considered as second-line treatment (Grade D1).68 A phase II clinical trial of tocilizumab, a humanized monoclonal antibody against the IL-6 receptor (NCT01727206), is currently underway.
Corticosteroids, cytotoxic chemotherapies, radiotherapy, and surgery.
Prior to the discovery of IFN-α as ECD treatment, many therapeutic regimens involving cytotoxic chemotherapies were reported in small series, including vinca alkaloids, anthracyclines, cyclophosphamide, and high-dose chemotherapy with autologous stem cell transplantation.69-76 Corticosteroids may reduce edema acutely, for example in severe exophthalmos, but are not considered effective monotherapy (Grade D2). The purine analog cladribine has been used in the treatment of multisystem LCH and both newly diagnosed and refractory ECD, although published reports of its efficacy are few.3,77,78 Use in the second-line setting should be considered (Grade D1). A prospective clinical trial of combined treatment with sirolimus and prednisone is currently underway (ACTRN12613001321730). Radiotherapy to ECD has been reported, although it has yielded no response or, at best, short-term palliation with disease progression within months.79,80 The role of surgical debulking is limited in ECD to severe orbital lesions or surgically resectable intracranial lesions.
Serine/threonine kinase inhibitors: vemurafenib and imatinib.
To date, treatment of 3 ECD patients has been reported with vemurafenib, an inhibitor of BRAF harboring the V600E mutation, with dramatic and unprecedented clinical and radiographic improvement.81 Clinical experience with vemurafenib in ECD is rapidly increasing, both in prospective clinical trials (NCT01524978 and a study at Hôpital Pitié-Salpêtrière, Paris, France) and in “off-label” administration. The responses reported in BRAF-mutant ECD to vemurafenib suggest that treatment with a BRAF inhibitor in a clinical trial should be pursued for all ECD patients requiring therapy and harboring the BRAFV600E mutation (Grade C2). There is currently insufficient clinical evidence to exclude all BRAFV600E-negative ECD patients from treatment with currently available RAF inhibitors, although this has yet to be attempted. Common toxicities include fatigue, arthralgias, headache, and multiple skin complications, including the development of squamous cell carcinomas. Because vemurafenib is not currently FDA approved for histiocytic disorders, enrollment in clinical trials rather than off-label use is encouraged. Critical questions such as the optimal duration of therapy, sequelae of drug discontinuation, and possible long-term effects of vemurafenib are currently unknown and important reasons for administration of drug in the context of a clinical trial.
Imatinib mesylate has been successfully used in case reports of other histiocytic disorders.82,83 Although no histiocytic disorders have known mutations in KIT, ABL, or PDGFR, some histiocytic lesions in ECD and related disorders appear to have abundant expression of PDGFR-β.84 Results in 7 patients treated with imatinib have been mixed, although it is a reasonable therapeutic strategy when first-line treatments have failed (Grade C0).84,85
Pediatric ECD.
As ECD is extremely rare in children, evidence regarding treatment is limited to case reports. Success with IFN-based therapies has been reported in single cases and with anakinra in one patient.66,86,87 Successful treatment with corticosteroid monotherapy has been reported twice.88,89 Of note, direct implementation of LCH-protocol therapies to children with ECD was unsuccessful in 2 cases.66,90
Disease surveillance, treatment duration, and prognosis
FDG-PET should be performed every 3 to 6 months for all patients following the initiation of treatment (Grade C0), and the interval between scans can be increased once disease has stabilized.91 Organ-specific imaging of affected organs should be performed every 3 months initially after beginning treatment, every 6 months once disease stabilization is achieved, or earlier as indicated by a change in clinical status or laboratory values such as renal function (Grade C2). There are no specific serum biomarkers of disease; C-reactive protein is elevated in 80% of cases at the time of diagnosis, and following its level may be helpful in monitoring treatment (Grade D1).15
Currently, it is recommended that treatment be continued indefinitely if tolerated; however, attempting treatment cessation for patients with a disease burden that is minimal or stable for a prolonged period of time may be reasonable on a case-by-case basis (Grade D2). Indefinite treatment with BRAF inhibition is potentially unsafe given the risk of accelerating premalignant RAS-mediated neoplastic lesions,92 although to date no treatment course has been defined for vemurafenib in ECD.
The prognosis of ECD has been reported as poor, with 43% of patients alive after average follow-up of 32 months.93 Recent reports describing survival of patients uniformly treated with interferon therapy describe an overall 5-year survival of 68% in ECD.45 The informal experience of the authors is that long-term survival is currently even more promising, although this impression is not reflected in the recent literature.
Conclusion
ECD is a rare, multi-system disorder requiring multidisciplinary collaboration in its diagnosis and treatment. Recent studies have made progress in redefining the pathogenesis of this disorder and establishing molecularly and immunologically based targeted therapeutics. Biopsy demonstrating characteristic histopathologic features in addition to clinico-radiographic features, most often sclerosing long bone involvement, is required to establish a diagnosis. A number of laboratory and radiographic analyses are needed upon diagnosis to ascertain the baseline extent of disease. In addition, mutational analysis establishing BRAF and RAS mutational status is critical in all ECD patients, even if this requires an additional biopsy, as these bear implications for therapy with BRAF inhibition. Therapy is recommended at diagnosis in all patients, except for those patients with minimally symptomatic disease. Referral to an academic medical center with expertise in treating ECD is strongly recommended given the sparse clinical experience with this disorder. Prospective therapeutic trials are essential to furthering therapeutic progress in ECD.
There is an Inside Blood Commentary on this article in this issue.
The online version of this article contains a data supplement.
Acknowledgments
We thank Kathy Brewer, Christina Baker, and other members of the Erdheim-Chester Disease Global Alliance as well as all of the patients who have contributed to the knowledge essential in drafting these guidelines.
J.H., L.A., E.D., O.A.-W., and D.H. are supported by funding from the ECD Global Alliance. L.D. is supported by funding from the Italian Ministry of Health (GR-2009-1594586). E.D., O.A.-W., and D.H. are supported by the Geoffrey Beene Cancer Research Foundation.
Authorship
Contribution: All authors participated in outlining the manuscript and providing expert recommendation grading; E.L.D., D.M.H., and O.A.-W. wrote the manuscript with substantial input from L.A. and J.H.; all authors then contributed to editing of the manuscript; T.C. provided histopathologic figures; and E.L.D. provided radiographic images.
Conflict-of-interest disclosure: J.H. received honoraria from Glaxo Smith Kline for counseling of patients with histiocytosis on the treatments with targeted therapies. The remaining authors declare no competing financial interests.
Correspondence: Eli L. Diamond, Department of Neurology, Box 52, 1275 York Ave, New York, NY 10065; e-mail: diamone1@mskcc.org.
References
Author notes
E.L.D. and L.D. contributed equally to this study.
L.A. and J.H. contributed equally to this study.