Key Points
On the basis of its immunophenotype and gene expression profile, B-PLL may be considered a specific subgroup of MCL.
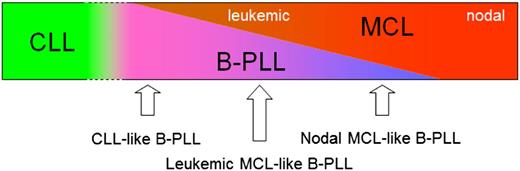
B-PLL is part of a spectrum ranging from CLL-like B-PLL, to leukemic MCL-like B-PLL, to nodal MCL-like B-PLL.
Abstract
B-cell prolymphocytic leukemia (B-PLL) is a rare mature B-cell malignancy that may be hard to distinguish from mantle cell lymphoma (MCL) and chronic lymphocytic leukemia (CLL). B-PLL cases with a t(11;14) were redefined as MCL in the World Health Organization 2008 classification. We evaluated 13 B-PLL patients [7 being t(11;14)-positive (B-PLL+) and 6 negative (B-PLL−)] and compared them with MCL and CLL patients. EuroFlow-based immunophenotyping showed significant overlap between B-PLL+ and B-PLL−, as well as between B-PLL and MCL, whereas CLL clustered separately. Immunogenotyping showed specific IGHV gene usage partly resembling MCL. Gene expression profiling showed no separation between B-PLL+ and B-PLL− but identified 3 subgroups. One B-PLL subgroup clustered close to CLL and another subgroup clustered with leukemic MCL; both were associated with prolonged survival. A third subgroup clustered close to nodal MCL and was associated with short survival. Gene expression profiles of both B-PLL+ and B-PLL− showed best resemblance with normal immunoglobulin M-only B-cells. Our data confirm that B-PLL+ is highly comparable to MCL, indicate that B-PLL− also may be considered as a specific subgroup of MCL, and suggest that B-PLL is part of a spectrum, ranging from CLL-like B-PLL, to leukemic MCL-like B-PLL, to nodal MCL-like B-PLL.
Introduction
B-cell prolymphocytic leukemia (B-PLL) is a rare disease, making up less than 1% of mature B-cell malignancies.1 B-PLL generally occurs in elderly people (median age at diagnosis, 69 years) and is characterized by the presence of more than 55% prolymphocytes in the peripheral blood (PB), no or minimal lymphadenopathy, massive splenomegaly, and very high white blood cell counts. The prognosis of B-PLL patients is generally poor, with a median survival of 3 years, although a subset of patients may show prolonged survival.1,2
Several studies have shown that in more than 20% of patients originally diagnosed with B-PLL, a translocation t(11;14)(q13;q32) involving the IGH and CCND1 genes is detectable. B-PLL patients with t(11;14) appeared to be of younger age and showed male predominance, extranodal involvement, and a slightly different immunophenotype (CD5+ and strong surface membrane [Sm] immunoglobulin [Ig] expression) compared with patients lacking this translocation.3 As t(11;14) is characteristic for mantle cell lymphoma (MCL), it was suggested that B-PLL with this translocation may represent a splenomegalic form of MCL evolving with leukemia.3,4 As a consequence, B-PLL with t(11;14) is now considered to be MCL in the most recent World Health Organization classification (WHO; 2008).5
The diagnosis of B-PLL is mainly based on clinical and morphological data (ie, >55% prolymphocytes).1
Correctly diagnosing the patient can, however, be difficult, as B-PLL has similarities with other mature B-cell malignancies; not only MCL but also rare cases of chronic lymphocytic leukemia (CLL), hairy cell leukemia-variant, and splenic marginal zone lymphoma.6-8 Although immunophenotyping may support a diagnosis of B-PLL, a B-PLL-specific immunophenotype has not been identified yet. Nevertheless, several new markers have recently been reported for the diagnosis and classification of mature B-cell malignancies,9 and it may well be that these markers also contribute to the diagnosis and classification of B-PLL.
In this study, we retrospectively analyzed 13 patients originally diagnosed with B-PLL, 7 of whom were t(11;14)-positive and thus classified as MCL according to the most recent WHO criteria, and compared them with leukemic MCL and CLL patients. EuroFlow-based 8-color immunophenotyping and gene expression profiling were performed to evaluate to what extent these patient groups differed and whether t(11;14)-positive B-PLL patients would indeed be more appropriately classified as MCL.
Material and methods
Patients
Thirteen patients with B-PLL, diagnosed between 1982 and 2010, were retrospectively included in this study on the basis of the availability of sufficient cell material. Patient characteristics are shown in Table 1 and supplemental Table 1. Diagnosis was based on clinical features, PB morphology, and immunophenotyping according to the WHO 2001 criteria.10 The diagnosis of the 6 patients who presented before 2001 were revised according to these criteria. In 7 patients, fluorescence in situ hybridization showed a t(11;14) translocation. Hence, these patients would now have been diagnosed as MCL according to WHO 2008 criteria.5
Patient characteristics
| Patient . | Sex (M/F) . | Age at diagnosis, years . | t(11;14) . | White blood cells ×109/L . | L . | H . | S . | Therapy* . | Survival, months† . |
|---|---|---|---|---|---|---|---|---|---|
| B-PLL 1 | M | 61 | No | 39 | No | No | Yes | Chemo | 135+ |
| B-PLL 2 | F | 59 | No | 30 | No | No | Yes | Chemo | 5 |
| B-PLL 3 | M | 70 | No | 173 | No | No | Yes | Chemo | 39 |
| B-PLL 4 | F | 84 | No | 180 | No | No | Yes | Chemo | 14 |
| B-PLL 5 | M | 49 | No | 53 | Yes | Yes | Yes | Chemo, SCT | 199+ |
| B-PLL 6 | M | 43 | No | 210 | Yes | No | Yes | Chemo | 97 |
| B-PLL 7 | M | 69 | Yes | 30 | No | No | Yes | Splen | 2.5 |
| B-PLL 8 | F | 74 | Yes | 6 | Yes | No | Yes | Splen, chemo | 108 |
| B-PLL 9 | M | 60 | Yes | 227 | No | No | Yes | Splen, chemo | 82+‡ |
| B-PLL 10 | M | 43 | Yes | 192 | Yes | No | Yes | Splen, chemo | 6 |
| B-PLL 11 | F | 63 | Yes | 37 | No | No | Yes | Splen, chemo | 19 |
| B-PLL 12 | M | 52 | Yes | 241 | Yes | Yes | Yes | Chemo | 12 |
| B-PLL 13 | F | 44 | Yes | 116 | No | Yes | Yes | Splen, chemo | 24 |
| Patient . | Sex (M/F) . | Age at diagnosis, years . | t(11;14) . | White blood cells ×109/L . | L . | H . | S . | Therapy* . | Survival, months† . |
|---|---|---|---|---|---|---|---|---|---|
| B-PLL 1 | M | 61 | No | 39 | No | No | Yes | Chemo | 135+ |
| B-PLL 2 | F | 59 | No | 30 | No | No | Yes | Chemo | 5 |
| B-PLL 3 | M | 70 | No | 173 | No | No | Yes | Chemo | 39 |
| B-PLL 4 | F | 84 | No | 180 | No | No | Yes | Chemo | 14 |
| B-PLL 5 | M | 49 | No | 53 | Yes | Yes | Yes | Chemo, SCT | 199+ |
| B-PLL 6 | M | 43 | No | 210 | Yes | No | Yes | Chemo | 97 |
| B-PLL 7 | M | 69 | Yes | 30 | No | No | Yes | Splen | 2.5 |
| B-PLL 8 | F | 74 | Yes | 6 | Yes | No | Yes | Splen, chemo | 108 |
| B-PLL 9 | M | 60 | Yes | 227 | No | No | Yes | Splen, chemo | 82+‡ |
| B-PLL 10 | M | 43 | Yes | 192 | Yes | No | Yes | Splen, chemo | 6 |
| B-PLL 11 | F | 63 | Yes | 37 | No | No | Yes | Splen, chemo | 19 |
| B-PLL 12 | M | 52 | Yes | 241 | Yes | Yes | Yes | Chemo | 12 |
| B-PLL 13 | F | 44 | Yes | 116 | No | Yes | Yes | Splen, chemo | 24 |
Chemo, chemotherapy (fludarabine [8 patients]; fludarabine, cyclophosphamide, rituximab [2 patients]; alemtuzumab [1 patient]; CHOP: cyclophosphamide, doxorubicin, vincristine, prednisone [6 patients]; DHAP: dexamethasone, cytarabine, cisplatin [3 patients]; chlorambucil [3 patients]; deoxycoformycin [1 patient]; high-dose cytarabine [1 patient]); H, hepatomegaly; L, lymphadenopathy;); S, splenomegaly; Splen, splenectomy; SCT, allogeneic sibling stem cell transplantation.
Splenectomy was particularly performed in patients diagnosed before 2001, when chemotherapeutic options to reduce spleen size were still limited. Spleen size was comparable between patients diagnosed before or after 2001. Morphological data from the removed spleen were available from patients 7, 8, 10, 11, and 13. B-PLL10 showed disrupted architecture with infiltration of prolymphocyte-like cells in particularly the red pulpa. B-PLL7 and B-PLL8 showed disrupted architecture with infiltration of MCL-like cells (either classical or monocytoid). B-PLL11 showed normal splenic architecture without clear leukemic infiltration. B-PLL13 showed normal splenic architecture with focal infiltration of atypical lymphocytes (some with a clear nucleolus), also in the red pulpa.
+, Still alive at last follow-up (2013).
Subsequently lost to follow-up.
Nine leukemic MCL patients and 7 CLL patients (supplemental Table 2, available on the Blood Web site) were retrospectively selected for this study on the basis of available diagnostic cell material. All CLL samples were obtained from PB, whereas the MCL samples were obtained from PB (n = 4; cases 2, 3, 7, and 8), BM (n = 2; cases 4 and 5), or lymph node (LN; n = 3; cases 1, 6, and 9). In addition, 27 MCL patients and 28 CLL patients, for whom flow cytometric and cytogenetic data were available, were included in part of this study (supplemental Table 3).
This study was approved by the Medical Ethics Committee of the Erasmus Medical Center (MEC-2013-444). This study was conducted in accordance with the Declaration of Helsinki.
Normal B-cell subsets
PB from healthy adults and tonsils from children were obtained with informed consent and according to the guidelines of the Medical Ethics Committee of the Erasmus Medical Center. PB mononuclear cells were enriched for B cells by magnetic separation with CD19 beads (Miltenyi Biotech) before purification of 5 IgM+ B-cell subsets: CD24hiCD38hiCD27− transitional, CD24dimCD38dimCD5+CD27− prenaive, CD24dimCD38dimCD5-CD27− naive mature, CD27+IgM+IgD+ natural effector, and CD27+IgM+IgD− IgM-only B cells.11 CD19+CD38+IgD−CD77− centrocytes were isolated from childhood tonsil, as described previously.12
Cytomorphology
PB cell differentiation (200 cells) and/or BM cell differentiation (500 cells) were routinely performed. Medium-sized cells with a prominent, central nucleolus and light blue cytoplasm were designated prolymphocytes.5
Fluorescence in situ hybridization analysis
DNA fluorescence in situ hybridization was performed according to the manufacturer’s instructions on fresh or thawed mononuclear cells using probes specific for 11q13 and 14q32 (VYSIS LSI IGH/CCND1 XT dual color dual fusion probes; Abbott Laboratories). Other cytogenetic analyses are described in the supplemental data.
Immunoglobulin heavy chain analysis
Rearranged IGH genes were amplified and sequenced as previously described.13 Nucleotide sequences were aligned to current databases (IgBlast and IMGT/V-Quest),14 and the percentage identity to the most close germline IGHV gene was assessed by counting the number of mutated nucleotides form the first codon downstream of the forward primer in FR1 and the last codon of FR3.
Immunophenotyping
Cryopreserved mononuclear cells were thawed and stained with the EuroFlow B-CLPD panel of monoclonal antibodies according to the EuroFlow protocol.9,15 Cells were acquired on a FACSCanto II flow cytometer (BD Biosciences), and data were analyzed with Infinicyt software (Cytognos). Freezing and thawing had no effect on the immunophenotypic results (supplemental Figure 1).
Gene expression profiling
RNA was isolated from cryopreserved mononuclear cells. In cases with a tumor load less than 90% (as determined by immunophenotyping), tumor cells were first purified using a FACSDiva or FACSAria cell sorter (BD Biosciences) after staining with CD19-PE (4G7; BD Biosciences). The extracted RNA was converted to cDNA, and subsequently to biotinylated cRNA, as described.16 All cRNA samples were hybridized to Affymetrix HG-U133 Plus 2.0 GeneChip arrays (containing 54 675 probe sets).17,18
Robust multiarray average background removal, quantile normalization, and probe set summary were performed.19 Correlation plots were made on the basis of a selected subset of probe sets that showed signal (ie, for which the median absolute deviation from the median exceeded a certain threshold T on log2 scale). To assess differential expression, significance analysis of microarrays was applied.20 Fold change (FC) was analyzed between disease categories; in the text, “D” and “U” denote probe sets downregulated or upregulated, respectively, in the last-mentioned disease category relative to the first-mentioned disease category.
The accession number of the array data is E-MTAB-1991. The data can be found online at https://www.ebi.ac.uk/arrayexpress/experiments/E-MTAB-1991/.
RQ-PCR
Real-time quantitative-polymerase chain reaction (RQ-PCR) was performed on cDNA, as previously described.21 Primers were used in combination with the Universal Probe Library (Roche Applied Sciences; supplemental Table 4). The quality and quantity of the cDNA were determined by RQ-PCR analysis of the ABL gene.22
Statistical analyses
Statistical analyses were performed with the Mann-Whitney U test or χ2 test, as indicated in the text. P < .05 was considered statistically significant.
Results
Patients
In all 13 B-PLL patients, prolymphocytes were present in high percentages. No typical CLL cells or mantle cells were observed. All patients had splenomegaly, whereas lymphadenopathy was present in 5 patients and hepatomegaly was present in 3 patients (Table 1). Seven patients died within 3 years from diagnosis, whereas 6 patients showed prolonged survival. There were no obvious differences in clinical or laboratory parameters between t(11;14)-positive and t(11;14)-negative B-PLL patients or between patients with short survival and prolonged survival.
Immunogenotyping
IGH gene rearrangement analysis was performed to prove monoclonality and to evaluate whether the clonotypic IGH repertoire is skewed within B-PLL patients. Putatively functional IGH gene rearrangements were detected in all 13 B-PLL patients. In 11 patients, the rearrangement involved a member of the large IGHV3 and IGHV4 subgroups. The most frequently recurring IGHV gene was IGHV3-23, which was used in 3 t(11;14)-positive cases (Table 2). When taking 98% as a cutoff in analogy to CLL, 6 cases (46%) were unmutated, most of which (4/6) showed 100% identity. In the 7 mutated patients, the percentage identity ranged from 89.8% to 96.5% (mean, 93.2%). There were no significant differences in percentage of mutated cases (43% vs 67%) or in level of identity with germline (97.5% vs 94.7%) between t(11;14)-positive and t(11;14)-negative B-PLL patients (Table 2). There was no significant relation between the IGHV status and clinical outcome; all unmutated patients, however, died within 40 months of diagnosis.
Immunogenotype of B-PLL patients
| Patient . | t(11;14) . | IGH rearrangement* . | Percentage identity in IGHV . | ||
|---|---|---|---|---|---|
| IGHV . | IGHD . | IGHJ . | |||
| B-PLL1 | No | 3-9 | 3-16 | 4*02 | 92.2 |
| B-PLL2 | No | 3-53 | ND | 4*02 | 89.8 |
| B-PLL3 | No | 4-39 | 5-5 | 5*02 | 98.7 |
| B-PLL4 | No | 3-49 | 3-10 | 5*02 | 99.6 |
| B-PLL5 | No | 3-43 | 6-19 | 4*02 | 93.8 |
| B-PLL6 | No | 3-33 | ND | 6*02 | 93.8 |
| B-PLL7 | Yes | 3-23 | 6-6 | 4*02 | 100 |
| B-PLL8 | Yes | 6-1 | 4-17 | 4*02 | 96.5 |
| B-PLL9 | Yes | 4-61 | 4-17 | 4*02 | 95.7 |
| B-PLL10 | Yes | 4-59 | 1-20 | 3*02 | 90.5 |
| B-PLL11 | Yes | 3-23 | 4-11 | 4*02 | 100 |
| B-PLL12 | Yes | 1-8 | 1-1 | 5*02 | 100 |
| B-PLL13 | Yes | 3-23 | 3-9 | 4*02 | 100 |
| Patient . | t(11;14) . | IGH rearrangement* . | Percentage identity in IGHV . | ||
|---|---|---|---|---|---|
| IGHV . | IGHD . | IGHJ . | |||
| B-PLL1 | No | 3-9 | 3-16 | 4*02 | 92.2 |
| B-PLL2 | No | 3-53 | ND | 4*02 | 89.8 |
| B-PLL3 | No | 4-39 | 5-5 | 5*02 | 98.7 |
| B-PLL4 | No | 3-49 | 3-10 | 5*02 | 99.6 |
| B-PLL5 | No | 3-43 | 6-19 | 4*02 | 93.8 |
| B-PLL6 | No | 3-33 | ND | 6*02 | 93.8 |
| B-PLL7 | Yes | 3-23 | 6-6 | 4*02 | 100 |
| B-PLL8 | Yes | 6-1 | 4-17 | 4*02 | 96.5 |
| B-PLL9 | Yes | 4-61 | 4-17 | 4*02 | 95.7 |
| B-PLL10 | Yes | 4-59 | 1-20 | 3*02 | 90.5 |
| B-PLL11 | Yes | 3-23 | 4-11 | 4*02 | 100 |
| B-PLL12 | Yes | 1-8 | 1-1 | 5*02 | 100 |
| B-PLL13 | Yes | 3-23 | 3-9 | 4*02 | 100 |
ND, not detected.
In-frame productive IGH rearrangements are shown. The underlined IGHV genes are frequently used in MCL patients.41
Immunophenotyping
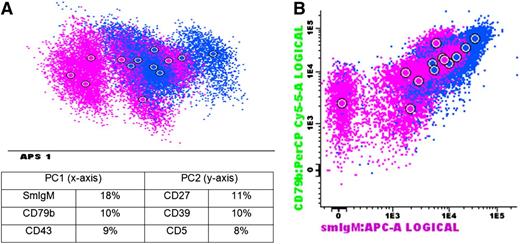
Detailed immunophenotyping of B-PLL patients showed a clonotypic immunoglobulin (Ig) light chain expression; positivity for CD19, CD20, CD22, HLA-DR, CD79b, and CD31; and negativity for CD23 in all cases, irrespective of t(11;14)-status (supplemental Figure 2). In contrast, CD27 was only expressed in 5 cases, all of which were t(11;14)-negative. IgM was positive in 12 of 13 cases and negative in only a single t(11;14)-negative B-PLL case (B-PLL1). Multiparametric analysis using Automated Population Separator (APS) plots showed a relatively heterogeneous pattern: B-PLL cases with a t(11;14) clustered somewhat apart from the t(11;14)-negative cases but without a clear separation (Figure 1). The most discriminatory markers in the APS plot were SmIgM, CD79b, and CD43 in principal component 1 (PC1), showing best separation, and CD39, CD27, and CD5 in PC2. Of note, SmIgM, CD79b, and CD43 were also the markers most heterogeneously expressed within MCL patients. In contrast, the most discriminatory markers within CLL patients were CD23, HLADR, and CD39 in PC1 and CD38, CD79b, and CD200 in PC2. These data indicate that SmIgM, CD79b, and CD43 are heterogeneously expressed in both B-PLL and MCL patients, but that these markers do not differ significantly within CLL patients.
Immunophenotype of B-PLL patients. (A) APS view of B-PLL patients. Below the APS plot, the most informative markers for the first (PC1, x-axis) and second (PC2, y-axis) PC are shown. (B) Dot plot of markers that were most discriminative in the APS view (2 top-ranked markers in PC1). Blue: B-PLL with t(11;14); purple: B-PLL without t(11;14).
Immunophenotype of B-PLL patients. (A) APS view of B-PLL patients. Below the APS plot, the most informative markers for the first (PC1, x-axis) and second (PC2, y-axis) PC are shown. (B) Dot plot of markers that were most discriminative in the APS view (2 top-ranked markers in PC1). Blue: B-PLL with t(11;14); purple: B-PLL without t(11;14).
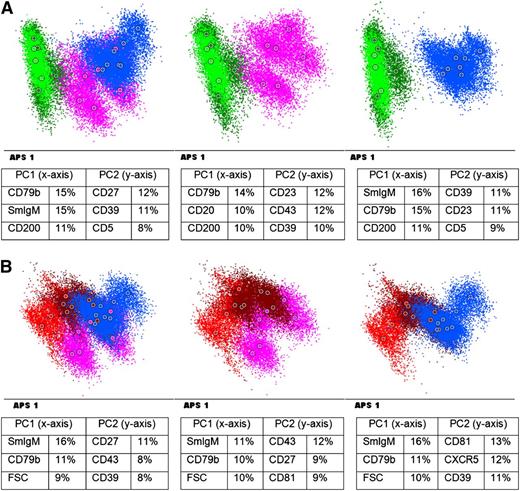
We next compared the immunophenotype of B-PLL patients with that of CLL and MCL patients. As shown in Figure 2A, a good separation of B-PLL and CLL cases was observed, with the most discriminatory markers being CD79b, SmIgM, and CD200 in PC1 and CD27, CD39, and CD5 in PC2. Two B-PLL patients [B-PLL5 and B-PLL6, both t(11;14)-negative] clustered separately but closer to the CLL patients (CLL-like B-PLL), whereas all other B-PLL patients were separated more clearly. In contrast, MCL and B-PLL cases showed considerable overlap, although some clustering was observed (Figure 2B). Of note, the 3 MCL cases obtained from LN seemed to cluster together at the end of the spectrum. The most discriminatory markers in the APS plot were SmIgM, CD79b, and CD43 in PC1 and CD27, CD39, and CD43 in PC2. The resemblance between MCL and B-PLL cases was supported by plotting the B-PLL cases in a fixed APS plot of the CLL and MCL cases, showing that all but 1 of the B-PLL cases was within the 2 standard deviation (SD) curves of the MCL cases (supplemental Figure 3). Only B-PLL1 was just outside the 2 SD curves but still clearly showed more similarities with MCL than with CLL cases.
Immunophenotype of B-PLL patients vs CLL patients and MCL patients (A) APS view of B-PLL patients vs CLL. (B) APS view of B-PLL patients vs MCL. Blue: B-PLL with t(11;14); purple: B-PLL without t(11;14);brown: MCL (obtained from BM/PB); red: MCL obtained from LN); green: CLL. Below each APS plot the most informative markers for the first (PC1) and second (PC2) PC are shown. FSC: forward scatter.
Immunophenotype of B-PLL patients vs CLL patients and MCL patients (A) APS view of B-PLL patients vs CLL. (B) APS view of B-PLL patients vs MCL. Blue: B-PLL with t(11;14); purple: B-PLL without t(11;14);brown: MCL (obtained from BM/PB); red: MCL obtained from LN); green: CLL. Below each APS plot the most informative markers for the first (PC1) and second (PC2) PC are shown. FSC: forward scatter.
Collectively, the immunophenotyping data show that B-PLL patients immunophenotypically form a spectrum ranging from cases close to but separate from CLL to cases clearly overlapping with MCL, and that there is no clear difference between t(11;14)-positive and t(11;14)-negative patients.
Because CLL and MCL are relatively heterogeneous entities, we aimed to confirm our data by comparing the 13 B-PLL patients with a larger set of 28 CLL patients (including all major cytogenetic subgroups as well as cases with high percentages of prolymphocytes) and 27 MCL patients. As shown in supplemental Figures 4 and 5, all but 1 of the CLL patients clustered clearly apart from B-PLL, whereas the MCL and B-PLL showed major overlap. The single CLL patient overlapping with B-PLL had 40% of prolymphocytes with atypical morphology, resembling MCL, but no t(11;14) was detected.
Finally, a comparison of our B-PLL patients with splenic marginal zone lymphoma patients, which also show splenomegaly and may clinically resemble PLL, showed clearly separate clusters in APS plots, indicating that both groups are immunophenotypically different (supplemental Figure 6).
Gene expression profiling
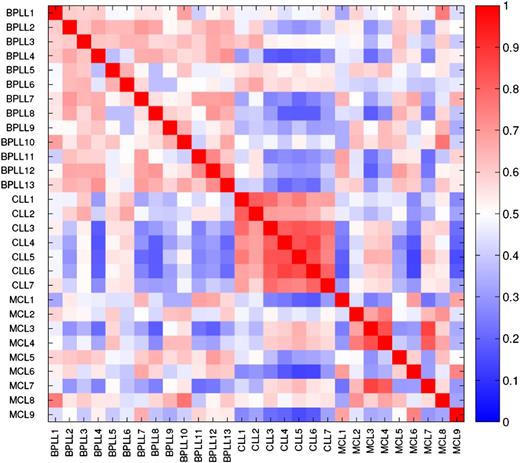
To get a general view of the gene expression data, correlation was visualized using a subset of 1269 probe sets that showed significant variation over all microarrays (median absolute deviation >0.8). The correlation plot showed clear homogeneity within the CLL patients, whereas B-PLL and MCL cases were more heterogeneous (Figure 3).
Correlation between microarray data derived from the individual patients, showing a well-defined CLL group, but no other correlations or clustering of the other patient groups.T = 0.8; 1340 probe sets.
Correlation between microarray data derived from the individual patients, showing a well-defined CLL group, but no other correlations or clustering of the other patient groups.T = 0.8; 1340 probe sets.
A comparison of the 3 patient categories (using significance analysis of microarrays analysis at a false discovery rate of 0.01) showed multiple genes being differentially expressed: 9822 probe sets between CLL and B-PLL, 953 between CLL and MCL, and 211 between B-PLL and MCL, confirming the APS-based immunophenotyping results that CLL is different from MCL and B-PLL and that the 2 latter diseases are much more comparable. Remarkably, comparison between t(11;14)-positive B-PLL and t(11;14)-negative B-PLL showed only 2 probe sets differentially expressed with a FC ≥2: CCND1 (FC: 66.9 D) and cartilage-associated protein (FC 2.3 D). MCL and t(11;14)-positive B-PLL showed differential expression (FC ≥2) of 14 probe sets, including RAB11A (FC: 2.5 D), RFP13 (FC: 2.1 D), and ABCC4 (FC: 2.5 D). Finally, comparison between t(11;14)-negative B-PLL and MCL showed 19 differentially expressed probe sets (FC ≥2), including CCND3 (FC: 2.3 D), ABCC4 (FC: 2.8 D), CCND1 (FC: 35.8 U), and bcl-1 (FC: 51.4 U; with the latter also encoding the CCND1 gene) (supplemental Table 5). Expression data of TP53, MYC, SOX11, CCND2, and CCND3, markers reported to potentially subdivide MCL,6,23-25 are shown in supplemental Table 6.
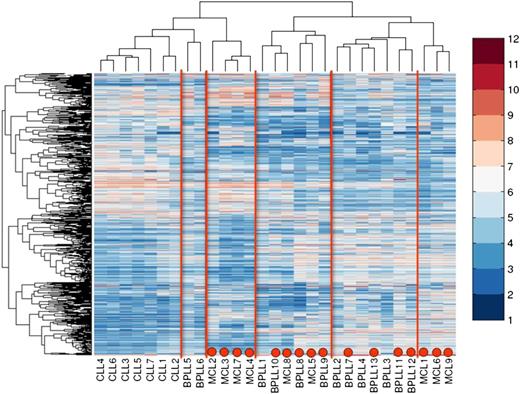
Unsupervised clustering of the microarray data identified at least 6 clusters (Figure 4). Cluster 1 contains all CLL cases. Cluster 2 is related to cluster 1 and consists of 2 B-PLL cases: B-PLL5 and B-PLL6, both of which are t(11;14)-negative and immunophenotypically CLL-like B-PLL. Both patients presented with lymphadenopathy and were still alive at last follow-up (>6 years). Clusters 3 and 6 only contain MCL cases, with all 3 MCL cases obtained from LN being present in cluster 6. Cluster 4 contains 2 MCL cases and 4 B-PLL cases (leukemic MCL-like B-PLL). Three of these 4 B-PLL patients were t(11;14)-positive, 2 had lymphadenopathy at diagnosis, and 3 patients survived for more than 6 years. Cluster 5 only contains 7 B-PLL patients and clustered closest to cluster 6 (nodal MCL-like B-PLL); 3 B-PLL were t(11;14)-positive, and 4 were t(11;14)-negative. Six of the 7 patients died within 2 years from diagnosis.
Unsupervised cluster analysis of gene-expression profiling data from B-PLL, MCL, and CLL patients. Red dots indicate a positive translocation t(11;14) status. T = 0.7; 2059 probe sets.
Unsupervised cluster analysis of gene-expression profiling data from B-PLL, MCL, and CLL patients. Red dots indicate a positive translocation t(11;14) status. T = 0.7; 2059 probe sets.
These data indicate that the gene expression profile of B-PLL is different from CLL. However, it shows considerable overlap with MCL, irrespective of t(11;14) status. It also indicates that B-PLL and MCL are both relatively heterogeneous diseases in which collectively at least 4 subtypes can be identified (leukemic MCL, nodal MCL, leukemic MCL-like B-PLL, and nodal MCL-like B-PLL).
Confirmation of differentially expressed genes
Twelve genes differentially expressed between any of the patient groups were subsequently selected for further analysis on the basis of fold change (≥2) and possible function. For 5 of these genes (CD23, CD200, CD49d, CD79b, CD22), the resulting protein products were already evaluated, using our immunophenotyping panel (supplemental Figure 7), whereas for the remaining 7 genes, RQ-PCR was performed (supplemental Figure 8). All selected genes from our microarray analysis were confirmed to be differentially expressed. Of note, with the exception of CCND1, none of the other markers was significantly differently expressed between t(11;14)-positive and t(11;14)-negative patients.
Comparison of B-PLL with normal B-cell subsets
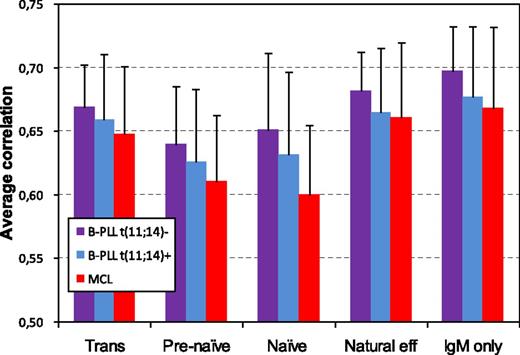
To obtain more insight into the origin and maturation status of B-PLL cells, gene expression profiling of B-PLL cells and 5 normal IgM+ B-cell subsets was performed. In an unsupervised clustering based on the probe sets that showed signal variation over all arrays, B-PLL cases clustered together and separately from the normal B-cell subsets (data not shown). Correlation analysis showed the highest level of correlation between B-PLL and memory B-cells (particularly IgM-only B-cells), both for t(11;14)-positive B-PLL as well as t(11;14)-negative B-PLL (Figure 5). Although B-PLL cases with unmutated IGHV genes correlated best with IgM-only B-cells as well, the level of correlation was higher for B-PLL cases with mutated IGHV genes (0.66 vs 0.71). In addition, MCL cases correlated best with the IgM+ memory B-cell subsets (Figure 5).
Correlation analysis between B-PLL cases and MCL cases vs 5 normal PB IgM+ B-cell subsets based on gene expression profiling.T = 0.8; 5194 probe sets.
Correlation analysis between B-PLL cases and MCL cases vs 5 normal PB IgM+ B-cell subsets based on gene expression profiling.T = 0.8; 5194 probe sets.
Discussion
Our data show that EuroFlow-based immunophenotyping and gene expression profiling separates B-PLL patients from CLL patients but that there is no clear separation from MCL. Although t(11;14)-positive B-PLL patients are now being considered as MCL, according to WHO 2008 criteria, our data do not show significant differences between patients with or without t(11;14), suggesting that B-PLL patients without t(11;14) also are closely related to MCL. On the basis of our data and data previously published by others, we hypothesize that B-PLL is part of a spectrum ranging from close to CLL to fully overlapping with MCL, resulting in CLL-like B-PLL, leukemic MCL-like B-PLL, and nodal MCL-like B-PLL (Figure 6).
Hypothetical model for the spectrum from CLL, B-PLL, and MCL. CLL is different from MCL and B-PLL. Some B-PLL cases, probably all t(11;14)-negative, have some similarities with CLL and may have a relatively good prognosis (CLL-like B-PLL). Another subgroup of B-PLL is indistinguishable from leukemic MCL patients (leukemic MCL-like B-PLL); this subgroup contains both t(11;14)-positive and t(11;14)-negative patients. A third B-PLL subgroup is comparable to but distinguishable from nodal MCL (nodal MCL-like B-PLL).
Hypothetical model for the spectrum from CLL, B-PLL, and MCL. CLL is different from MCL and B-PLL. Some B-PLL cases, probably all t(11;14)-negative, have some similarities with CLL and may have a relatively good prognosis (CLL-like B-PLL). Another subgroup of B-PLL is indistinguishable from leukemic MCL patients (leukemic MCL-like B-PLL); this subgroup contains both t(11;14)-positive and t(11;14)-negative patients. A third B-PLL subgroup is comparable to but distinguishable from nodal MCL (nodal MCL-like B-PLL).
B-PLL is rare and consequently only limited studies have focused on this disease. The hallmark of the disease is the presence of more than 55% prolymphocytes in PB, but several studies have shown that these cases include a spectrum of patients with de novo B-PLL, B-PLL occurring in patients with a previously well-established diagnosis of CLL, and t(11;14)-positive neoplasms, particularly MCL.4 Our data confirm that B-PLL is different from CLL, both by EuroFlow-based immunophenotyping and by gene expression profiling. However, a subgroup of t(11;14)-negative B-PLL patients with an immunophenotype and gene expression profile close to CLL might exist, which might be related to prolonged survival. A recent gene expression profiling study by the group of Catovsky and Matutes also showed that B-PLL and CLL are biologically different diseases, each showing a specific and homogeneous genomic signature.26 Of importance, many transcripts found to be overexpressed in B-PLL in their study were also identified in our study. Although the CLL cases included in our gene expression study did not show an increased percentage of prolymphocytes, such cases were included in the study by Del Giudice,26 suggesting that CLL with increased percentages of prolymphocytes also is biologically distinct from de novo B-PLL. In line with this, we show that CLL-PL cases generally cluster separately from B-PLL on the basis of immunophenotype.
Because t(11;14) is a characteristic cytogenetic abnormality in MCL and t(11;14)-positive B-PLL cases histopathologically or cytologically can resemble MCL, the t(11;14)-positive B-PLL cases are now classified as MCL according to WHO 2008.3,5 Our data confirm that t(11;14)-positive B-PLL and MCL are indeed very similar regarding their immunophenotype, immunogenotype, and gene expression profile. However, t(11;14)-negative B-PLL also appear very similar to t(11;14)-positive B-PLL and MCL, both immunophenotypically and transcriptionally, with the sole difference being the expression of CCND1. Of note, in an unsupervised analysis performed by Del Giudice and colleagues, one case, originally described as B-PLL and subsequently reclassified as MCL on the basis of t(11;14), clustered with other B-PLL.26 This observation is in line with our data showing high levels of similarity between t(11;14)-negative B-PLL and t(11;14)-positive B-PLL/MCL. Only a few genes were differentially expressed between t(11;14)-negative B-PLL and MCL. These genes included CCND1/bcl1, upregulated in MCL (as expected), and CCND3, upregulated in the t(11;14)-negative B-PLL. Of note, cyclin D1-negative MCL cases have been reported; these patients appear to be clinically and biologically similar to the conventional cyclin D1-positive MCL but frequently express cyclin D2 or cyclin D3 because of chromosomal rearrangements or epigenetic mechanisms.24,25,27,28 Our data suggest that the CCND3 gene may be the counterpart of CCND1 in t(11;14)-negative B-PLL patients, although the level of overexpression was relatively low, and that B-PLL might be a specific cyclin D1-negative subgroup of MCL. However, SOX11 expression, a highly specific marker for MCL that also identifies the cyclin D1-negative subtype,23 was highly expressed in most of our MCL cases, whereas the expression in the B-PLL patients was highly variable, irrespective of t(11;14) status (supplemental Table 6). Immunophenotypically, t(11;14)-positive and t(11;14)-negative B-PLL could not well be discriminated, although expression of particularly SmIgM, CD79b, CD27, and CD5 was somewhat different. Comparable results have been reported previously for CD5 and SmIg.3
Because our B-PLL patient series is relatively small, more subtle differences may not have been detected. Furthermore, the limited number of MCL patients included in our study likely does not fully represent the internal variability of this patient group.6,29,30 Rosenwald analyzed 92 MCL cases and identified 2 MCL subsets differing in cyclin D1 expression and survival.29 The authors were able to construct a model based on only 4 proliferation signature genes (CDC2, ASPM, tubulin-α, and CENP-F) that predicted length of survival. None of these genes differed in expression in our study between MCL and B-PLL, irrespective of t(11;14) status (significance analysis of microarrays, false-discovery rate (FDR) <0.01). Enhancement of cell proliferation in MCL was also shown in another study.31 Vizcarra and colleagues6 report that leukemic MCL patients could be divided in 2 subgroups: classical MCL with normal MYC and TP53 expression and positivity for CD38, and PLL-like cases with MYC amplification, loss of TP53, and no expression of CD38. The first group seems to correspond with clusters 4 and 5 in our study, whereas the classical MCL overlaps with cluster 6 (supplemental Table 6). Our gene expression data in MCL confirm that MCL is a heterogeneous disease and show that the gene expression profile of LN-derived MCL cells may be different from PB- or BM-derived MCL cells, as was also reported by Rubio-Moscardo et al.32 Part of our B-PLL cases (leukemic MCL-like B-PLL) clustered with the MCL cases derived from BM and PB, whereas other B-PLL cases (nodal MCL-like B-PLL) clustered separately and close to MCL obtained from LN (irrespective of t(11;14) status). Interestingly, most B-PLL cases related to leukemic MCL showed prolonged survival comparable to the rather indolent clinical course in leukemic MCL, whereas all but 1 of the B-PLL patients related to nodal MCL died within 2 years of diagnosis.
We identified several markers differentially expressed between CLL, MCL, and B-PLL. HDGFRP3, highly expressed in MCL, was recently identified as an inducer of proliferative arrest in MCL cells33 and is expected to promote cell proliferation. High EBF-1 expression (as found in MCL, and particularly B-PLL) was recently suggested to be involved in disease progression34 and was found to be downregulated in CLL.35 Other genes differentially expressed included markers related to the B-cell receptor complex (SmIgM, CD79b), several molecules involved in adhesion and migration (CD29, CD49d), and several activation/immune response genes (CD69, CD22). CD29 and CD49d together form the VLA-4 protein; CD49d is known to empower CLL cells to migrate into lymphoid tissue.36,37 CD69, an early activation marker in CLL correlating with a poor prognosis,38 was heterogeneously expressed in MCL and B-PLL. Altogether, the genes found to be differentially expressed among CLL, MCL, and B-PLL are in line with data reported for CLL and MCL in literature and thereby confirm the suitability of our approach. The cell surface markers that may discriminate B-PLL cells from MCL (IgM, CD79b, CD43, CD27, CD29, and CD69) may prove valuable for the diagnosis of B-PLL; this requires larger prospective international studies.
Analysis of IGH gene mutations and usage showed predominant usage of IGHV3 and IGHV4 genes. In line with previous reports,39,40 IGHV3-23 was most frequently used and about half of B-PLL cases showed an unmutated pattern.39 Interestingly, in MCL patients, the usage of IGHV3-23 is associated with more aggressive disease and more frequent infiltration of BM.41 Furthermore, in non-nodal MCL cases, the percentage of IGHV mutated patients is higher than in nodal MCL patients (56% vs 10%) and the percentage of identity to the most close germline IGHV gene is significantly lower.7 These data resemble our findings in B-PLL, in which SHM and IGHV3-23 usage were detected in the nodal-MCL-like cases only (see supplemental Table 7).
To define the normal counterpart of B-PLL cells, we compared the gene expression profile of B-PLL cases with sorted normal B-cell subsets. Because all but 1 of the B-PLL cases were IgM-positive, we focused on normal IgM-positive B-cells. B-PLL cases showed highest level of correlation with IgM-only cells, which are formed in germinal centers in response to T cell-dependent antigens. The finding that about half of the B-PLL cases had SHM in their IGHV genes is in line with a postgerminal center origin. Of note, CD27 was expressed on 5 of 6 t(11;14)-negative B-PLL cases but was absent on the 7 t(11;14)-positive B-PLL cases. Both groups nevertheless correlated best with IgM-only normal B cells when analyzed separately, indicating that the CD27-negative B-PLL cases also may originate from memory B-cells.
In conclusion, the high similarity in gene expression profile and immunophenotype between t(11;14)-positive B-PLL and MCL supports the WHO 2008 criteria in which both groups are considered as a single entity (MCL). In addition, the t(11;14)-negative B-PLL cases should not be considered a separate entity. In fact, our data indicate that B-PLL forms a specific and heterogeneous subgroup of MCL, ranging from close to CLL to full overlap with MCL: CLL-like B-PLL, leukemic MCL-like B-PLL, and nodal MCL-like B-PLL. Identifying well-defined subtypes in MCL will be particularly helpful in designing novel, risk-adapted treatment strategies for this heterogeneous disease.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
We thank Chantal Crama for technical assistance and Dr Peter Valk for supporting the bioinformatic analyses. We gratefully acknowledge Dr W.A.F. Marijt (Leiden University Medical Center, Leiden), Dr C. van der Heul (St. Elisabeth Hospital, Tilburg), and Dr M.R. Schipperus (Haga Hospital, The Hague) for providing clinical data.
This work was in part supported by an unrestricted grant from Roche.
Authorship
Contribution: V.H.J.v.d.V., K.v.L., and M.K.K. designed the study; P.G.H. and M.S.v.-d.S. performed the experiments; H.B.B. and K.v.L. performed the cytogenetic analyses; V.H.J.v.d.V., M.S.v.-d.S., D.d.R., and M.S. analyzed the data; V.H.J.v.d.V., M.C.v.Z., S.B., D.K., A.O., K.L., P.J.L., J.J.M.v.D., A.W.L., M.K.-K., and K.v.L. collected and interpreted the data; and V.H.J.v.d.V. wrote the manuscript. All authors approved the final version of the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: V.H.J. van der Velden, Department of Immunology, Wytemaweg 80, 3015 CN Rotterdam, The Netherlands; e-mail: v.h.j.vandervelden@erasmusmc.nl.
References
Author notes
M.K.-K. and K.v.L. contributed equally to this study.