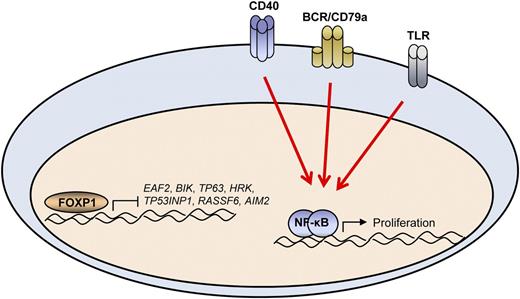
In this issue of Blood, van Keimpema et al present a novel study highlighting the role of FOXP1 in cooperation with nuclear factor κB (NF-κB) in the survival and proliferation of B cells.1
The FOXP1 and NF-κB pathways work together to promote the growth of normal primary B cells as well as DLBCL B cells. In DLBCL patients, overall survival can be determined by the expression of a subset of 7 FOXP1 target genes. In the absence of NF-κB signaling, the survival benefit of FOXP1 is lost, indicating the importance of this pathway is most relevant in cells that rely on signaling pathways such as CD40, B-cell receptor (BCR), and Toll-like receptor (TLR) to maintain constitutive NF-κB activation.
The FOXP1 and NF-κB pathways work together to promote the growth of normal primary B cells as well as DLBCL B cells. In DLBCL patients, overall survival can be determined by the expression of a subset of 7 FOXP1 target genes. In the absence of NF-κB signaling, the survival benefit of FOXP1 is lost, indicating the importance of this pathway is most relevant in cells that rely on signaling pathways such as CD40, B-cell receptor (BCR), and Toll-like receptor (TLR) to maintain constitutive NF-κB activation.
Diffuse large B-cell lymphoma (DLBCL) accounts for approximately 30% of all lymphomas, with an estimated 10 000 deaths annually in the United States.2,3 It results from uncontrolled growth of B lymphocytes, and it is typically classified into 2 main subgroups based on the resemblance to an activated B cell (ABC) or a germinal center B cell (GCB).4
Agents targeting signaling components of the B-cell receptor (BCR) pathway have produced excellent responses in chronic lymphocytic leukemia and in some types of ABC DLBCL.5 This response is attributed to the fact that these cells exhibit chronic BCR signaling, which causes them to respond to agents that target NF-κB.6 However, there remain few therapeutic options for GCB or ABC lymphomas that do not respond to initial therapy or that eventually relapse. The difficulty in treating DLBCL is in part due to the heterogeneity of the disease, with several active pathways and various mutations that contribute to pathogenesis. A better understanding of the pathogenic and cell-survival mechanisms in DLBCL is critical to identify new molecular targets for therapy.
Proteins of the forkhead box (FOX) family of transcription factors are involved in cell growth and differentiation, and as such, several members of this family have been implicated in cancer development.7 FOXP1 specifically is a poor prognostic factor in DLBCL8 and may predict transformation to an aggressive lymphoma.9 In their Blood article, van Keimpema et al highlight the antiapoptotic property of FOXP1. They determined relevant FOXP1 target genes by microarray analysis after overexpressing or knocking down FOXP1 in primary B cells from healthy donors as well as DLBCL cell lines, and the predominant pathway identified in these studies is the apoptosis pathway. A set of 7 genes repressed by FOXP1 was further analyzed in a large set of 498 DLBCL patients on a clinical trial evaluating rituximab plus CHOP, and lower expression of this gene set was found to correlate with shorter overall survival and progression-free survival, which was irrespective of ABC vs GCB subtype. Therefore, lower expression of these genes, presumably due to higher FOXP1 expression, is indicative of a poor prognosis.
The authors performed functional studies to corroborate the role in apoptosis, which is indicated by the gene expression studies. They show that overexpression of FOXP1 in primary B cells (in the presence of CD40 ligand stimulation) was able to promote B-cell survival (indicated by decreased caspase-3/7 activation), whereas small interfering RNA knockdown of FOXP1 in DLBCL cell lines increased apoptotic cell death. There was no impact on cellular proliferation (determined by carboxyfluorescein diacetate succinimidyl ester dilution assays) upon knockdown of FOXP1, indicating that the role of FOXP1 is primarily on cell viability.
Because the role of FOXP1 was determined in cell lines cultured with CD40L, which can activate NF-κB, the authors went on to determine the contribution of NF-κB to the FOXP1-mediated survival. FOXP1 had no effect in cells without NF-κB activation (provided by either CD40L or constitutively active IKK protein, CA-IKK2). However, coexpression of FOXP1 and CA-IKK2 protein verifies that NF-κB provides a proliferative effect whereas FOXP1 provides an antiapoptotic effect. These 2 parallel pathways work together to maintain the increased number of primary B cells in culture. This also suggests that therapeutically disrupting FOXP1 function may be expected to have a stronger effect in ABC DLBCL, which has been shown to have a strong dependence on NF-κB signaling.
In summary, these studies further define the mechanism of FOXP1-mediated B-cell survival and the role of NF-κB. The authors identify a set of 7 FOXP1 target genes that clearly correlate with survival in DLBCL patients and therefore may be useful as a diagnostic tool. Because this work uncovers a clear survival benefit with activation of both FOXP1 and NF-κB together over either pathway alone, this opens the door to therapeutic combination strategies in lymphoma.
Conflict-of-interest disclosure: The author declares no competing financial interests.