Abstract
Background: Disease relapse and graft vs host disease (GvHD) following alloHSCT are major causes of treatment (Tx) failure in patients (pts) with MDS and AML (Paietta, 2012). Studies of parenteral azacitidine, an epigenetic modifier, have shown efficacy in preventing post-transplant relapse in MDS and AML pts, and possibly reducing GvHD severity (Platzbecker, 2012; de Lima, 2010). Azacitidine maintenance after alloHSCT may enhance graft vs leukemia (GvL) effects and reduce GvHD by expansion of regulatory T cells (Goodyear, 2010, 2012). CC-486 is a novel oral formulation of azacitidine which, as well as potentially allowing easier administration over extended schedules, could increase the duration of drug exposure to residual malignant cells. We now report preliminary results from a prospective phase I/II dose-finding study of CC-486 as maintenance Tx after alloHSCT in pts with MDS or AML.
Objective: To evaluate the maximum tolerated dose (MTD), dose-limiting toxicities (DLT), pharmacokinetics (PK), and safety outcomes of CC-486 following alloHSCT in pts with MDS or AML.
Methods: Pts with WHO-defined MDS or AML who have undergone alloHSCT with myeloablative (MAC) or reduced-intensity conditioning (RIC) regimens and who had sibling or unrelated donors with ≤1 antigen mismatch at the HLA-A, -B, -C, -DRB1 or -DQB1 locus, are enrolled. CC-486 Tx is initiated between days (d) 42 and 84 after alloHSCT. Pts receive prophylactic Tx for GvHD and infections per institutional standard. A standard 3x3 MTD design is being used to evaluate 4 dosing schedules: CC-486 200 mg or 300 mg QD, administered for 7 d (Cohorts 1 and 2) or 14 d (Cohorts 3 and 4) of repeated 28d Tx cycles. MTD determination is based on DLT that occur during the first 2 Tx cycles (pts are not evaluable if unable to complete 2 Tx cycles for reasons other than a DLT). Adverse events (AEs) are graded using NCI-CTCAE v4.0. Pts are followed for safety, incidence of acute and chronic GvHD, and relapse. PK of azacitidine after CC-486 administration, alone or with standard concomitant medications, are evaluated on d 1 of CC-486 Tx cycles 1 and 2.
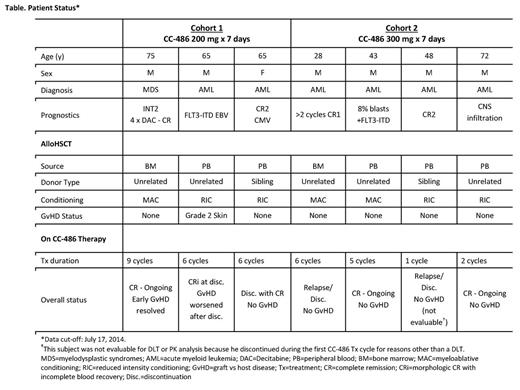
Results: At data cut-off (7/17/2014), outcome data were available for 7 pts enrolled in the first 2 cohorts (Table): 1 pt had IPSS Int-2 MDS and 6 had AML with high-risk features. Stem cell source was from bone marrow (n=2) or peripheral blood (n=5) from unrelated (n=5) or sibling (n=2) donors. Two pts had 1 antigen mismatch and 5 had a full match.Conditioning included MAC (n=3) and RIC (n=4) regimens. One pt in Cohort 2 was not evaluable for DLT or PK assessments due to early discontinuation (AML relapse) during the first CC-486 Tx cycle. Of the 6 evaluable pts, 4 completed ≥6 CC-486 Tx cycles and 2 ongoing pts had not reached 6 cycles on-study at data cut-off (Table). One pt who had GvHD before CC-486 Tx subsequently withdrew from the study after developing febrile neutropenia and diarrhea related to GvHD of the bowel; 1 pt withdrew for personal reasons; and 1 pt discontinued after 6 Tx cycles due to relapse. At data cut-off, 3 pts remained on-study, all with continued CR (Table). No pt in Cohort 2 developed GvHD. For all pts, most AEs were grade 1-2. Grade 3-4 AEs were reported for 2 pts: both had nausea and vomiting, which were controlled with antiemetic agents upon onset; 1 also had device-related infection and dyspnea, and 1 also had febrile neutropenia and rash. No DLT emerged with these CC-486 regimens and no pt experienced secondary graft failure. Overall azacitidine plasma concentration profiles (Figure) and PK parameters were similar following CC-486 given alone or with concomitant medications, and were within range of values observed following similar CC-486 doses in pts with MDS, CMML, and AML (Garcia-Manero, 2011). The AUC range for oral CC-486 is ~10% of that seen with subcutaneous azacitidine administered at 75 mg/m2 (Garcia-Manero, 2011).
Conclusion: Preliminary data from this analysis suggest that CC-486 administered at doses of 200 or 300 mg QD for 7 days every 4 weeks is safe and well tolerated in the post-transplant setting. Overall, azacitidine plasma concentration profiles and PK parameters were not affected by use of concomitant medications. No DLT and a low rate of GvHD support the ongoing evaluation of 14d extended CC-486 dosing regimens in this ongoing study, as a preliminary to a prospective, randomized trial of this new agent post-transplant.
de Lima:Celgene: Consultancy. Champlin:Celgene: Consultancy. Giralt:Celgene: Consultancy, Honoraria, Research Funding, Speakers Bureau. Scott:Celgene: Consultancy, Honoraria, Research Funding. Hetzer:Celgene: Employment, Equity Ownership. Wang:Celgene: Employment, Equity Ownership. Laille:Celgene: Employment, Equity Ownership. Skikne:Celgene: Employment, Equity Ownership. Craddock:Celgene: Honoraria, Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.


This feature is available to Subscribers Only
Sign In or Create an Account Close Modal