Abstract
Mantle cell lymphoma (MCL) is an aggressive B-cell lymphoma that frequently demonstrates chemoresistance. Since a number of signaling pathways are dysregulated in MCL, novel strategies for restoring multiple tumor suppressors and pathways are of considerable interest.
Exportin 1 (XPO1/CRM1) mediates nuclear export of numerous molecules, including oncogenic transcription factors, ribosomal subunits, and RNAs, and is critical for cancer survival and proliferation. We previously reported that single-agent XPO1 antagonist KPT-185 exhibited antiproliferative and proapoptotic activities against MCL cells via inhibiting synthesis of proteins, such as chaperone proteins (HSP70), through ribosomal biogenesis and via nuclear export of transcription factors and oncogenic mRNAs, including cyclin D1, c-Myc, and PIM1 (Tabe et al. ASH 2013). Intriguingly, proteomic analysis detected significant upregulation of glycolysis and gluconeogenesis pathways in KPT-185–treated MCL cells. Aerobic glycolysis plays an important role in sustaining tumor metabolism and may negatively affect the antitumor activity of KPT-185. We therefore assessed the efficacy of combining this regulator of nucleocytoplasmic shuttling with an inhibitor of mTOR signaling, which is a central regulator of cell metabolism integrating nutrients, with KPT-185 targeting the altered metabolism.
We first investigated the antitumor effects and molecular mechanisms of simultaneous treatment with KPT-185 and the ATP-competitive second-generation mTOR kinase inhibitor AZD-2014 in three MCL cell lines: JVM2, Jeko-1, and MINO (KPT-185 IC50 values: 92, 103 and 96 nM, respectively, at 48 h by MTT). AZD-2014 treatment resulted in downregulation of p-S6K and c-Myc and upregulation of p27KIP and cleaved caspase-9, which translated into concentration-dependent reduction of cell proliferation (IC50: JVM2, 247 nM; Jeko-1, 86 nM; MINO, 370 nM, at 48 h by MTT). The KPT-185/AZD-2014 combination inhibited cell growth (% of control absorbance; values given are for KPT-185 [100nM], AZD-2014 [100nM for JVM2 and MINO, 50nM for Jeko-1], and KPT-185/AZD-2014: JVM2 49.4±2.0, 61.6±3.7, 25.2±0.2; Jeko-1 46.7±4.8, 66.9±3.3, 28.6±3.4; MINO 55.0±5.6, 79.6±0.6, 21.6±2.5, at 48 h by MTT).
We next investigated changes of protein expression and signaling pathways induced by AZD-2014 or the KPT-185/AZD-2014 combination (24 h) in Jeko-1 cells (KPT-100nM, AZD-2014 200nM). The proteomic technology of isobaric tags for relative and absolute quantitation (iTRAQ) demonstrated that AZD-2014 affected expression of 68 proteins (42 upregulated / 26 downregulated) and caused downregulation of fatty acid synthase expression (P<0.001). We also observed repression of importin-9, a transporter from cytoplasm to nucleus, by AZD-2014 (P<0.05) and of exportin-1, a transporter from nucleus to cytoplasm by KPT-185/AZD-2014 (P<0.001), indicating that the combination of KPT-185/AZD-2014 may disrupt bidirectional nucleocytoplasmic shuttling in MCL cells.
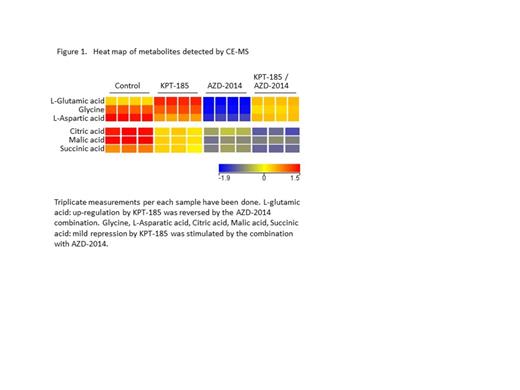
We then performed comprehensive and quantitative analysis of charged metabolites by capillary electrophoresis mass spectrometry in Jeko-1 cells after treatment with KPT-185 or KPT-185/AZD-2014 (24 h) to detect differences in 52 polar metabolites (P<0.05). We observed that KPT-185–stimulated glutamate metabolism was effectively reversed by AZD-2014 (fold change of L-glutamic acid compared to control: KPT-185, 1.3; AZD-2014, 0.5; KPT185/AZD-2014, 0.8; P<0.001, contol vs KPT185/AZD-2014). AZD-2014 enhanced the repression of fatty acid synthesis by KPT-185 (0.3 fold) with significant downregulation of citric acid (0.3 fold, P<0.001). KPT-185/AZD-2014 combination further decreased succinic acid (0.04 fold, P<0.001, compared to control) and malic acid (0.1 fold, P<0.001), and both of these effects were associated with gluconeogenesis downregulation (Figure 1).
Taken together, our findings suggest that inhibition of mTOR kinase enhances the antitumor effects of the XPO1 antagonist KPT-185 with effective repression of XPO1 blockage–induced glycolysis/gluconeogenesis upregulation and of fatty acid synthesis and with possible disruption of bidirectional nucleocytoplasmic shuttling in MCL cells. These findings suggest a novel, rationally designed combinatorial strategy targeting pro-survival metabolism in MCL.
Andreeff:Karyopharm: Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal