Abstract
Background: Janus kinases (JAKs), including JAK1 and JAK2, mediate the signaling of cytokines and growth factors implicated in the pathogenesis of myelofibrosis (MF). Suppression of JAK2 leads to cytopenias due to its involvement in the signaling pathways of thrombopoietin and erythropoietin.
Purpose: The objective of this ongoing study is to evaluate the efficacy and safety of INCB039110, a selective JAK1 inhibitor, in patients with MF with the goal of improving MF-related symptoms with less myelosuppression than seen with JAK1/JAK2 inhibition. Here, we report the 12- and 24-week efficacy and safety of INCB039110 in a phase II trial.
Methods: Adults with intermediate-1 or higher (per Dynamic International Prognostic Scoring System [DIPSS]) primary MF (PMF), post–polycythemia vera MF (PPV-MF), or post–essential thrombocythemia MF (PET-MF) were eligible regardless of JAK2V617F mutation status. A platelet count of ≥ 50 × 109/L, hemoglobin ≥ 8.0 g/dL (transfusions permitted), and a palpable spleen or prior splenectomy were required.
Patients assessed the severity of 19 disease-related symptoms daily using the modified Myelofibrosis Symptom Assessment Form v3.0 electronic diary. Spleen volume (SV) was evaluated by magnetic resonance imaging or computed tomography at baseline, week 12, and week 24. The primary endpoint was the proportion of patients with a ≥ 50% reduction from baseline in total symptom score (TSS, consisting of the sum of 6 individual symptom scores: night sweats, itchiness, abdominal discomfort, pain under left ribs, early satiety, and bone/muscle pain) at week 12. Other endpoints included the proportion of patients with a ≥ 50% reduction from baseline in TSS at week 24, percentage change from baseline in TSS at week 12 and 24, percentage change from baseline in SV at week 12 and 24, and safety.
The study used a Simon 2-stage design to assess 3 separate dose cohorts (100 mg twice daily [BID], 200 mg BID, and 600 mg once daily [QD]). A dose cohort could be expanded if ≥ 3 of the first 10 patients met the primary endpoint (intent-to-treat method).
Results: Enrollment is complete and 87 patients have been treated with INCB039110: 10 in the 100 mg BID, 45 in the 200 mg BID, and 32 in the 600 mg QD groups; 10, 42, and 31 patients, respectively, were evaluable for the primary endpoint. The 200 mg BID and 600 mg QD cohorts met criteria for expansion.
Enrolled patients (mean age, 64 years) had PMF (55%), PPV-MF (26%), or PET-MF (18%), and most had intermediate-1 (37%) or intermediate-2 (47%) risk by DIPSS. Mean SV at baseline was 2442.7 cm3, mean hemoglobin was 10.2 g/dL, and mean platelet count was 246.7 × 109/L.
Reductions in TSS were similar between the 200 mg BID and 600 mg QD groups, and largely maintained through week 24 (Table). Modest reductions in spleen volume were attained in the 200 mg BID and 600 mg QD groups.
| INCB039110 Dose | |||
| 100 mg BID | 200 mg BID | 600 mg QD | |
| Patients with ≥ 50% improvement in TSS,* n/N (%) | |||
| Week 12 | 2/10 (20) | 15/42 (36) | 10/31 (32) |
| Week 24 | 2/10 (20) | 12/42 (29) | 11/31 (35) |
| Median change from baseline in TSS,† % | |||
| Week 12 | −28.5 | −45.8 | −37.2 |
| Week 24 | −57.2 | −48.6 | −46.7 |
| Median change in SV,† % | |||
| Week 12 | −0.5 | −14.1 | −14.5 |
| Week 24 | −31.1 | −17.4 | −17.1 |
| * Patients who discontinued prior to the week 12 or 24 visit were considered nonresponders at that time point. † Only patients with baseline and week 12 or 24 data were included. Negative change = improvement. | |||
| INCB039110 Dose | |||
| 100 mg BID | 200 mg BID | 600 mg QD | |
| Patients with ≥ 50% improvement in TSS,* n/N (%) | |||
| Week 12 | 2/10 (20) | 15/42 (36) | 10/31 (32) |
| Week 24 | 2/10 (20) | 12/42 (29) | 11/31 (35) |
| Median change from baseline in TSS,† % | |||
| Week 12 | −28.5 | −45.8 | −37.2 |
| Week 24 | −57.2 | −48.6 | −46.7 |
| Median change in SV,† % | |||
| Week 12 | −0.5 | −14.1 | −14.5 |
| Week 24 | −31.1 | −17.4 | −17.1 |
| * Patients who discontinued prior to the week 12 or 24 visit were considered nonresponders at that time point. † Only patients with baseline and week 12 or 24 data were included. Negative change = improvement. | |||
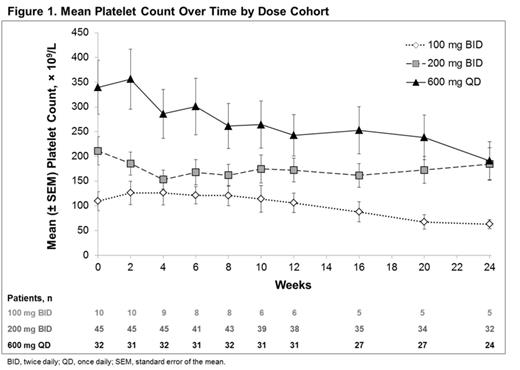
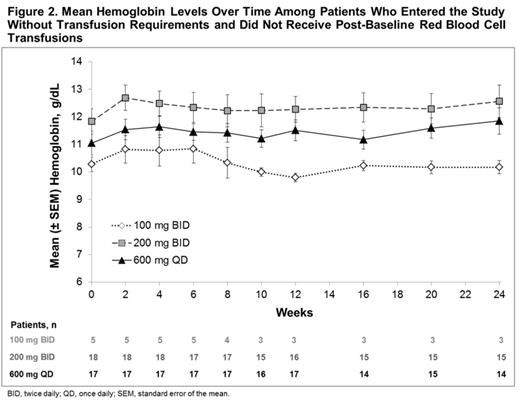
Mean platelet count is shown in Figure 1. Mean hemoglobin levels in patients who entered the study without transfusion requirements and did not receive post-baseline blood transfusions are shown in Figure 2. In these patients, the mean percent change from baseline in hemoglobin at week 24 increased by 5.6% in the 200 mg BID group and 8.6% in the 600 mg QD group.
The most common nonhematologic adverse events (occurring in > 15% of enrolled patients overall regardless of causality) were fatigue (29%), nausea (21%), upper respiratory tract infection (18%), constipation (17%), diarrhea (17%), and cough (16%); most of these events were grade 1 or 2 and did not appear to be dose dependent. New or worsening grade 3 or 4 anemia occurred in 33% and 0% of patients, respectively, and thrombocytopenia in 24% and 5% of patients, respectively.
Conclusions: Patients with MF treated with the JAK1 inhibitor INCB039110 (200 mg BID or 600 mg QD) continued to show meaningful improvements in MF-related symptoms and modest decreases in spleen size, while preserving mean hemoglobin levels over time through week 24.
Mascarenhas:Incyte Corporation: Consultancy. Talpaz:ARIAD, BMS, Sanofi. Incyte, Pfizer: Research Funding. Gupta:Novartis: Consultancy, Honoraria, Research Funding; Incyte Corporation: Consultancy, Research Funding. Foltz:Novartis: Consultancy, Honoraria, Research Funding; Incyte Corporation: Research Funding; Gilead: Research Funding; Promedior: Research Funding; Janssen: Consultancy. Savona:Karyopharm: Consultancy, Equity Ownership, Membership on an entity's Board of Directors or advisory committees; Gilead : Membership on an entity's Board of Directors or advisory committees; Incyte Corporation: Membership on an entity's Board of Directors or advisory committees; Celgene : Membership on an entity's Board of Directors or advisory committees. Paquette:Incyte Corporation: Speakers Bureau. Coughlin:Bayer: Honoraria, Membership on an entity's Board of Directors or advisory committees; Amgen: Honoraria, Membership on an entity's Board of Directors or advisory committees; Eastern Health: Employment. Winton:Incyte Corporation: Membership on an entity's Board of Directors or advisory committees, Research Funding. Hunter:Incyte Corporation: Employment. Assad:Incyte Corporation: Employment. Clark:Incyte Corporation: Employment. O'Neill:Incyte Corporation: Employment. Hoffman:All Cells LLC: Consultancy, Membership on an entity's Board of Directors or advisory committees; Geron: Consultancy, Membership on an entity's Board of Directors or advisory committees. Verstovsek:Incyte Corporation: Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal