Abstract
The breakthrough clinical success of immune checkpoint inhibition with PD-1 and CTLA-4 antibodies illustrates the potential of T cell based immunotherapy to effectively treat malignancies. However, a remaining major challenge is to increase the specificity and frequency of anti-cancer immune responses. A rational approach to achieve this goal is therapeutic vaccination, as highlighted by a recent phase II vaccination study using naturally presented HLA ligands, which induced vaccine-specific immune responses that were associated with improved clinical outcome (Walter et al., Nat. Med. 2012). This underscores the potential of identifying patho-physiologically relevant targets by direct differential analysis of the entire landscape of HLA-presented peptides, termed the HLA ligandome.
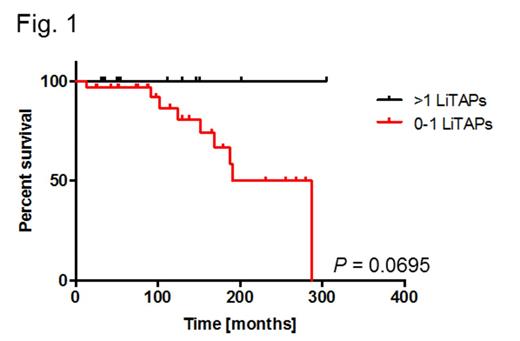
Here we applied this approach to chronic lymphocytic leukemia (CLL) with the aim of developing a CLL-specific multi-peptide vaccine. Using cohorts of 30 patients and 30 healthy volunteers, we comparatively and extensively mapped the HLA ligandome landscape of CLL and identified more than 20,000 different HLA class I and II ligands representing 7,377 source proteins, attaining >95% of the maximum attainable coverage (as extrapolated from saturation analysis). A set of 49 HLA ligand source proteins (225 different HLA ligands) showed CLL-exclusive representation in >20% of CLL patients, thereby constituting the novel category of ligandome-derived tumor associated antigens (LiTAAs). These LiTAAs were validated to be broadly and frequently represented across different stages and mutational subtypes of CLL and found to be robustly represented in HLA ligandomes of patients undergoing standard chemo-/immunotherapy. Functional characterization by IFNγ-ELISPOT revealed peptide-specific immune recognition of 14/15 (93.3%) corresponding HLA ligands (LiTAPs) exclusively in CLL patients. These immune responses were strictly CLL (LiTAP-) directed and mediated by functional patient-derived CD8+ T cells. Notably, for immunoreactive LITAPs, a linear correlation between frequencies of representation in the CLL ligandomes and immune recognition by CLL patient PBMC was observed (R²=0.59), suggesting that LiTAP presentation on cancer cells is a direct prerequisite for the presence of anti-LiTAP immune responses. Moreover, we investigated the prognostic relevance of LiTAP-specific immune responses: Retrospective survival analysis of 45 CLL patients analyzed by IFNγ-ELISPOT assays comparing cases with T cell responses specific for 0-1 LiTAPs (n=32) versus >1 LiTAPs (n=13) suggests a prolonged overall survival in the multiple-response cohort as compared to the single/non-responders (P=0.0695, log-rank test, Fig. 1). Taken together these data provide clear evidence for tumor-dependent priming of LiTAP-specific T cells in CLL patients and indicate their involvement in tumor control in vivo. This directly implies these non-mutant self-peptides as pathophysiologically relevant tumor antigens in CLL thereby validating our target identification approach and encouraging future evaluation in clinical vaccination trials.
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal