Abstract
Introduction: Acute myeloid leukemia (AML) with inv(3)(q21q26) or t(3;3)(q21;q26) [inv(3)/t(3;3)] is associated with aberrant expression of the stem cell regulator EVI1 and dismal prognosis. Recently, we and others (Gröschel et al, Cell, 2014; Yamazaki et al, Cancer Cell, 2014) have shown, as a consequence of the inv(3)/t(3;3) rearrangements, that the proto-oncogene EVI1 is activated upon the structural repositioning of a distal GATA2 enhancer from 3q21 to EVI1, coinciding with loss of GATA2 expression from the rearranged allele. Notably, GATA2 deficiency has been shown to impair hematopoietic stem cell frequency and function (Lim et al, J. Clin. Invest., 2012) and Evi1 activation in inv(3) murine models is followed by leukemia onset after a long latency of 6 months (Yamazaki et al). We therefore hypothesize that additional cooperating genetic lesions, other than EVI1 activation and GATA2 deregulation, are required for full leukemic transformation. We sought to extend the molecular characterization of inv(3)/t(3;3) myeloid malignancies through next-generation sequencing.
Methods: We selected 32 AML (including cell lines MUTZ-3 and UCSD-AML1), 4 CML-BC (including cell lines HNT-34 and MOLM-1), and 5 myelodysplastic syndrome (MDS) cases with inv(3)/t(3;3) rearrangements and performed whole transcriptome sequencing (n=40) as well as whole exome sequencing (WES, n=10) on the Illumina HiSeq 2500. For WES germline control we used DNA from cultured CD3+ T-cells from diagnostic bone marrow specimens. Variants were examined when recurrently detected in more than two patients or previously associated with leukemogenesis or cancer pathogenesis. Gene expression profiles (GEP) were constructed for 24 cases for subsequent analyses.
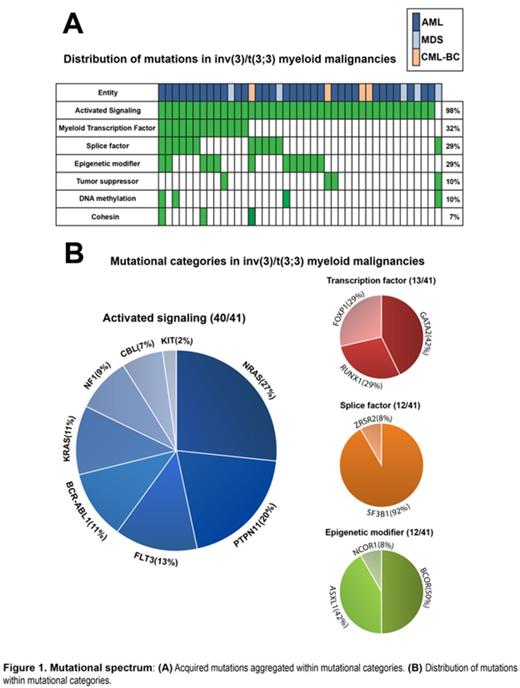
Results: All examined variants, validated by Sanger sequencing, were assigned to mutational categories to discern patterns of mutations (Figure 1A). We observed that all 41 cases acquired at least one mutation belonging to the mutational categories reported as relevant for leukemia pathogenesis. Importantly, all AML and CML-BC patients, as well as 4 out of 5 MDS patients contained mutations in genes activating RAS/RTK signaling, amounting to 98% of all inv(3)/t(3;3) myeloid malignancies. An aggregate of 74% of all patients harbor mutually exclusive mutations in RAS-pathway associated genes; NRAS(27%), PTPN11(20%), KRAS(11%), and NF1(9%). Additionally, we found mutations in genes affecting signaling pathways involving RAS; FLT3(13%), BCR-ABL(11%), and KIT(2%) (Figure 1B). NPM1, CEBPA, and IDH1/2 mutations were absent in all cases. Furthermore, frequent mutations were found in transcription factor (32%), splice factor (29%), epigenetic modifier (29%), and tumor suppressor (10%) encoding genes (Figure 1A). GATA2 is the most commonly mutated transcription factor in inv(3)/t(3;3) myeloid malignancies (15%). As these mutations were found on the non-rearranged GATA2 allele, only mutant GATA2 is expressed due to GATA2 silencing mediated by the 3q21q26 rearrangement. Novel somatic truncating mutations and copy number loss were detected in the transcription factor FOXP1(10%). Additionally, we observed frequent mutations in the splice factor gene SF3B1(27%) in AML and MDS cases. Finally, the predominant monosomal karyotype within inv(3)/t(3;3) myeloid malignancies is contrasted by the low incidence of TP53 mutations (5%). No mutational pattern alluded to the high coincidence of monosomy 7 (68%), but inv(3)/t(3;3)/-7 cases were characterized by a strong down regulation of the transcription factor CUX1.
In an effort to discriminate MDS and AML with inv(3)/t(3;3) we performed cluster and principle component analyses, revealing no cluster formation. Additionally, differential expression analysis was unable to differentiate AML from MDS, indicating strong homogeneity based on GEPs.
Conclusion: Myeloid malignancies with inv(3)/t(3;3) harbor a common set of molecular alterations, i.e. EVI1 activation and GATA2 deregulation, conjugated by mutations activating signaling pathways. Mutational patterns and GEPs precluded the discrimination of MDS and AML with inv(3)/t(3;3), supporting the notion that these myeloid disorders should be categorized as AML irrespective of blast count. Exploiting signaling pathways therapeutically may prove a valuable adjunct to the scarce armamentarium of drugs effective in this malignancy.
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal