Abstract
Recent randomised trials have suggested that intensification of daunorubicin in induction with a higher dose (90mg/m2) vs standard dose (45mg/m2) resulted in improved remission rate and overall survival. Apart from concerns about the outcome of the comparator arm in younger patients, exploratory subgroup analysis suggested that the benefit was seen in patients <50yrs, platelet counts >50, WBC <10 and intermediate cytogenetics while in older patients there was a survival benefit only in patients aged 60-65yrs, and a beneficial trend in core binding factor leukaemia. However, 60mg/m2 is widely used. In a retrospective comparison of non-contemporaneous patients no difference was found between a 90mg and a 60mg dose level. It has also been suggested that the beneficial effect of gemtuzumab ozogamicin in CBF leukaemia could be mimicked by a higher Daunorubicin dose. To clarify these issues we undertook the first randomised trial of 90mg/m2 vs 60mg/m2.
As part of the UK NCRI AML17 trial patients were randomised in a 1:1 fashion to either 90mg/m2 or 60mg/m2 on days 1,3 and 5 of their first induction course, followed by 50mg/m2 on days 1,3 and 5 in course 2. Ara-C was given 100mg/m2 12 hourly days 1-10 (course 1) and days 1-8 (course 2). Between 09/2011 and 10/2013, 1206 patients were randomised. The median age was 53yrs (16-72); 54% were male; 84% had de novo AML, 10% secondary, and 6% high risk MDS; median presenting WBC was 8.5 (0.3-430); 10% had favourable cytogenetics; 72% intermediate and 18% adverse. The distribution of genotype was: FLT3+/NPM1cwt 7%/ FLT3+/NPM1c+ 11%/ FLT3-/NPM1cwt 64%/ FLT3-/NPM1c+ 18%. There was no difference in characteristics between the arms.
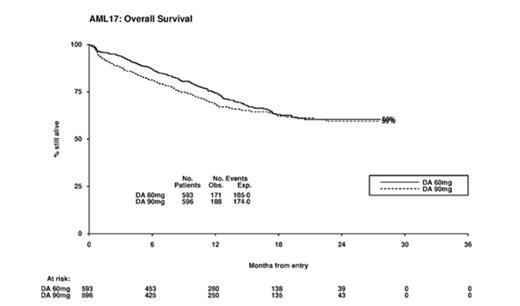
Results: There was no difference in remission rate between the arms (90mg/m2 81% vs 60mg/m2 84%. OR 1.21(0.90-1.64) p=0.1). The 30 and 60 day mortality was 6% vs 4% and 10% vs 5% (30-day HR 1.57 (0.94-2.61) p=0.09; 60-day HR 1.98 (1.30-3.02) p=0.001). Causes of 60-day mortality by treatment arm were: infection 25 vs 11; infection+haemorrhage 1 vs 3; haemorrhage 5 vs 3; resistant disease 14 vs 2; pulmonary 2 vs 4; cardiac 1 vs 2, multiple 5 vs 3; and unknown 5 vs 1.The 2-year RFS was 52% vs 50%, HR 1.06 (0.85-1.32) p=0.6; cumulative incidence of relapse was 37% vs 41%, HR 1.01 (0.79-1.30) p=0.9. With excess early mortality, and no corresponding improvement in RFS, survival in the 90mg arm was non-significantly worse (2-year OS 59% vs 60%; HR 1.17 (0.95-1.44) p=0.14). Beyond day 60, there was no difference in survival (HR 0.99 (0.78-1.25) p=0.9)
30% of patients were transplanted (169/604 in 90mg/m2; 197/602 in 60mg/m2, p=0.07; with 271 transplants in CR1 (123 vs 148), and 56 in CR2 (28 vs 28); 314 allografts were performed, 234 in CR1). When the data is censored at SCT results remain consistent (60% vs 61%; HR 1.21 (0.96-1.52) p=0.10).
Stratified analyses involving Age, Sex, Diagnosis (de Novo/Secondary/MDS), Cytogenetic group, Performance Status, ITD/NPM1 genotype was undertaken to identify whether any subgroups might show benefit for the 90mg dose. There was evidence of interaction between treatment and cytogenetics (p=0.01 for heterogeneity) and also with FLT-3 ITD status (p=0.03 for heterogeneity). However, in no subgroup was there a significant benefit for 90mg/m2. There was no benefit (90 vs 60) within the favourable (87% vs 100%) or intermediate risk (65% vs 64%) and adverse (24% vs 28%) risk groups, thus the suggestion that the favourable risk group might benefit based on the GO experience was not substantiated. Similarly, while there was a non-significant benefit for patients who were FLT-3 mutant (HR 0.74 (0.47-1.17) p=0.2) the survival was significantly worse in patient who were FLT3-ITD wild type (HR 1.31 (1.03-1.67) p=0.03). In particular, looking at patients with either age <50, WBC<10 or platelets >50, there was no evidence of either benefit for 90mg/m2 or heterogeneity of treatment effect. In the 304 patients >60 yrs. the OS was identical (HR 1.00 (0.69-1.45)) including for those aged 60-65yrs (HR 1.05 (0.69-1.61)). Supportive care in courses 1 or 2 were not significantly different between the two arms, and haematological recovery times were similar between the two arms.
Conclusion: In this first randomised comparison of Daunorubicin 90mg/m2 vs 60mg/m2 there was no survival benefit overall or in any of the subgroups examined for the 90mg/m2 dose level.
(We are grateful to Cancer Research UK for research support for this trial)
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.
This icon denotes a clinically relevant abstract

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal