Abstract
Background: Patients (pts) with advanced systemic mastocytosis (SM), including aggressive SM (ASM) and mast cell leukemia (MCL) ± associated clonal hematologic non–mast cell lineage disease (AHNMD), have a poor prognosis with few effective treatment options. More than 80% of pts express the activating KIT D816V mutation. The oral multikinase inhibitor midostaurin inhibits wild-type and D816-mutated KIT. In this global phase 2 trial (NCT00782067), the largest prospective trial in advanced SM, a planned interim analysis of stage 1 pts demonstrated a high overall response rate (ORR) with reductions in marrow mast cell (MC) burden and a favorable safety profile (Gotlib et al, Blood 2012). Updated stage 1 results showed improvements in symptoms and quality of life (QOL) (Gotlib et al, Blood 2013). Here, we report primary results for the fully accrued trial.
Methods: This single-arm trial consisted of an adapted Fleming 2-stage design. Midostaurin 100 mg twice daily was administered in continuous 28-day cycles until progression or unacceptable toxicity. Eligibility and responses (modified from Valent et al,Leuk Res 2003) were adjudicated by study steering committee (SSC). Histopathology was centrally assessed. Eligible pts had ≥ 1 measurable C-finding (CF) defined as SM-related organ damage. The primary endpoint was ORR (major response [MR] + partial response [PR]) occurring in the first 6 cycles and maintained for ≥ 8 weeks (wk).
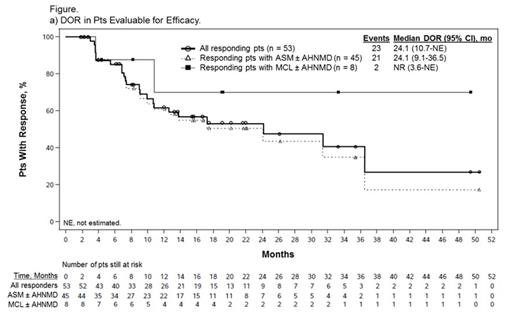
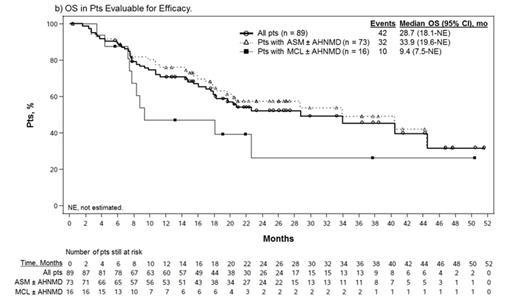
Results: Of 116 enrolled pts, 89 (77%) were eligible for efficacy evaluation; pts were considered ineligible by the SSC due to absence of measurable CF (n = 14) or organ damage considered unrelated to SM (n = 13). Median age was 64 years (range 25-82). Overall, 73 pts (82%) had ASM, including 57 (78%) with an AHNMD (ASM-AHNMD); 16 (18%) had MCL, including 6 (38%) with an AHNMD (MCL-AHNMD). AHNMDs included chronic myelomonocytic leukemia (n = 25), myelodysplastic/myeloproliferative neoplasm unclassifiable (n = 22), hypereosinophilic syndrome/chronic eosinophilic leukemia (HES/CEL; n = 5), and 11 other subtypes. A total of 37 pts (42%) had received ≥ 1 prior therapy (median 1; range 1-4). Seventy-seven pts (87%) were positive and 10 pts (11%) were negative for KIT D816V/Y/L mutation; 2 pts were not evaluable. The ORR was 60% (53/89); most responses were MRs (40/53, 75%), subtyped as incomplete remission (IR) in 36%, pure clinical response in 28%, and unspecified in 11%. PRs included good PR (GPR) in 21% and minor PR in 4% of pts. Overall, 12%, 11%, and 17% of pts had stable disease, progressive disease, or were not evaluable for response, respectively. After a median follow-up of 26 months (mo; range 12-54), the median duration of response (DOR) and median overall survival (OS) were 24.1 and 28.7 mo, respectively (Figure). Median OS in responders was 44.4 mo. Of 16 MCL pts, 8 responded, including 7 MRs (44%); median DOR was not reached (NR), with 3 IRs ongoing (49+, 33+, and 19+ mo). Median OS was 9.4 mo among all pts with MCL and NR among responders. Median change in MC burden in 72 evaluable pts was -59% (range -96% to 160%); 41 pts (57%) had ≥ 50% reduction. Median best change in serum tryptase level in 89 evaluable pts was -58% (range -99% to 185%); 32 pts (36%) had ≥ 50% reduction lasting ≥ 8 wk. Four of 5 responding ASM/MCL-HES/CEL pts (1 IR, 3 GPR) also had complete resolution of eosinophilia (mean % and absolute eosinophils at baseline: 57% and 12.4 × 109/L). Improvements in symptoms and QOL were consistent with those reported previously (Gotlib et al, Blood 2013).
All 116 pts received ≥ 1 midostaurin dose and were evaluable for safety. Grade 3/4 drug-related hematologic adverse events (AEs) were neutropenia (5%), leukopenia (4%), febrile neutropenia (3%), anemia (3%), and thrombocytopenia (3%). Low-grade nausea was common but usually manageable with antiemetics. The most frequent grade 3/4 drug-related non-hematologic AEs included nausea (6%), vomiting (6%), fatigue (4%), and increased lipase/amylase (4%/3%). As of July 9, 2013, 65 pts had discontinued therapy, most commonly due to progression (n = 31) and AEs (n = 18; 11 were considered drug related [3 hematologic and 8 non-hematologic AEs]). Seven patients developed acute myeloid leukemia related to a prior AHNMD.
Conclusions: Midostaurin exhibits a high rate of durable responses in pts with advanced SM, particularly in MCL, which has a dismal prognosis. The reductions in MC burden suggest that midostaurin may impact the natural history of the disease.
Gotlib:Novartis Pharmaceuticals Corporation: Membership on an entity's Board of Directors or advisory committees, Research Funding, Travel Support Other. George:Novartis: Consultancy, Membership on an entity's Board of Directors or advisory committees. Akin:Novartis: Consultancy. Sotlar:Novartis Pharma: Laboratory Services, Laboratory Services Other; Nanostring Technologies: Consultancy. Hermine:Novartis: Research Funding. Awan:Boehringer Ingelheim: Consultancy; Lymphoma Research Foundation: Research Funding. Mauro:Ariad: Consultancy, Honoraria, Research Funding, Speakers Bureau; Bristol Myers Squibb: Consultancy, Honoraria, Research Funding; Novartis Oncology: Consultancy, Honoraria, Research Funding; Pfizer: Consultancy, Honoraria, Speakers Bureau. Sternberg:Novartis Pharmaceuticals Corporation: Employment, Equity Ownership. Villeneuve:Novartis Pharma AG: Employment. Emery-Salbert:Novartis: Employment. Stanek:Novartis Pharmaceuticals Corporation: Employment. Hartmann:Novartis: Consultancy. Horny:Novartis: Consultancy. Valent:Novartis: Consultancy, Honoraria, Research Funding. Reiter:Novartis: Consultancy, Honoraria.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal