Abstract
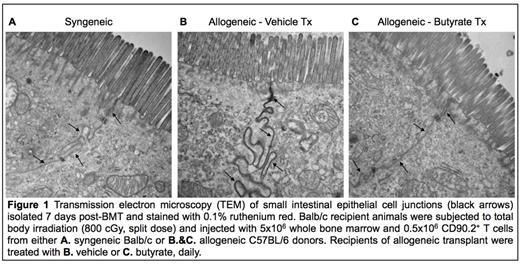
Alterations in the gastrointestinal (GI) microbiota have recently been implicated in the severity of graft versus host disease (GVHD). While most studies are focused on annotating the distinct microbial taxonomic compositions and/or the recognition of bacterial molecules by the immune system, little is known about the microbial metabolite composition (crucial for achieving the effects of the microbiota) on the GI epithelium and immune responses after allogeneic BMT. In order to profile the microbial metabolite composition in an unbiased way following allogeneic BMT, weutilized a clinically relevant MHC disparate (B6→BALB/c) model of GVHD and determined the metabolites by performing gas chromatography-mass spectrometry (GC-MS/MS) in a blinded manner. The syngeneic (BALB/c→BALB/c) animals and the naive untransplanted, age-matched BALB/c animals treated with similar diet, served as controls. We made the surprising observation that on day +7 (relative to transplant) there were no statistically significant differences in either the luminal contents of the small bowel or large bowel or in the plasma and spleens of the allogeneic animals when compared to the control animals. However, the short chain fatty acids (SCFA) derived from the microbiota, specifically butyrate, was significantly less only in the intestinal epithelial cells (IECs) of allo-recipients (P<0.03). These surprising results were validated by repeating the unbiased profiling studies on day +21. Additionally, the IECs (CD326+) harvested from the allo-animals on day +7 demonstrated reduced expression of SLC5A8 and GPR43 (P<0.015), which were recently recognized as receptors for butyrate. Because butyrate is known to inhibit histone deacetylases and alter the epigenome through histone acetylation, we next hypothesized that reduced butyrate levels within the IECs would affect the IEC histone acetylation. Consistent with the hypothesis, the (CD326+) IECs from the allo-recipients demonstrated significantly reduced histone H4-acetylation (P<0.02) on day +7. To determine whether butyrate has any direct functional effects, we harvested primary IECs from B6 animals, incubated them with butyrate, and next used them as targets in a cytotoxic assay with allo-stimulated BALB/c CD8+ T cells. Pretreatment of IECs with butyrate, enhanced their histone acetylation and their ability to resist apoptosis (P<0.04) induced by the allo-T cells, demonstrating direct functional relevance of butyrate on IECs. Next, to determine the potential in vivo relevance of reduced butyrate in IECs, and to determine if diminished butyrate absorption could be overcome in vivo, we administered butyrate (10mg/kg) daily from day 0 through 21 to the recipients of a B6→BALB/c model BMT. Intra-gastric administration of butyrate to allo-recipients significantly enhanced the acetylation of histone-H4 (P<0.003). Importantly, examination of the IECs on day +7 post-BMT with transmission electron microscopy (TEM), demonstrated significantly improved junction integrity in allo-recipients treated with butyrate (Fig. 1C), compared to vehicle treated controls (Fig. 1B) demonstrating direct effects on preservation of GI epithelial integrity.
Further, immunophenotype analyses of the donor T cells in the GI tract and the spleen on day +7 and +21 demonstrated a significant decrease in the total number of activated donor T cells (CD4+CD69+ & CD4+CD44hi) and an increase in the ratio of donor T regulatory cells (FoxP3+/CD44hi+) only on day +21 and not day +7, suggesting that this could be an indirect consequence of the reduction of IEC damage by butyrate observed at earlier time-point, day +7. Consistent with these observations, butyrate treatment resulted in the increased survival of the allogeneic animals (P<0.04). Similar protection from GVHD was also observed in a second, B6→B6D2F1 model of BMT demonstrating strain independent effects. Collectively our results demonstrate for the first time that (a) alterations of microbial metabolites, specifically the SCFAs that have direct and profound effects on the GVHD target tissue, GI epithelium and (b) suggest that enhancing the local microbial metabolite, butyrate, could be a potential novel strategy for mitigating GVHD.
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal