Abstract
Introduction: Acute myeloid leukemia (AML) is characterized by dysregulated gene expression and aberrant DNA methylation status. One alternative treatment for AML is hypomethylating agents such as 5-Azacytidine (AZA) and Decitabine (DAC), but just around 30% of the patients obtain a clinical response. These agents are slow acting requiring 3-4 cycles to demonstrate some efficacy, for both reasons looking for biomarkers to identify sensitive patients before treatment could be very beneficial to the patients.
Objective: Design an ex-vivo model using the automated flow cytometry ExviTech® platform to identify the antiproliferative effect of hypomethylating agents such as 5-Azacytidine and Decitabine in leukemic cells from AML whole bone marrow patient samples. This assay may capture changes of hypomethylating agents effect that may potentially predict ex vivo the patient clinical outcome.
Methods: Bone-marrow samples from 10 patients diagnosed of AML were sent to Vivia from 24 hospitals across Spain within 24h. Whole sample were incubated for 72h in in well plates containing 8 concentrations of AZA and DAC. To induce blast proliferation, AML cells were resuspended in RPMI medium and human cytokines including GM-DSF, IL3, SCF, G-CSF, EPO and transferrin. The number of proliferative and no proliferative live leukemic cells was determined using the CFDA dye. Blast population and viability was determined labeling with monoclonal antibodies and Annexin V. Dose-response curves of AZA and DEC were measured in these 10 patient samples.
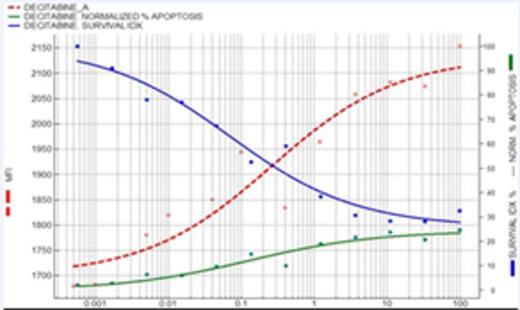
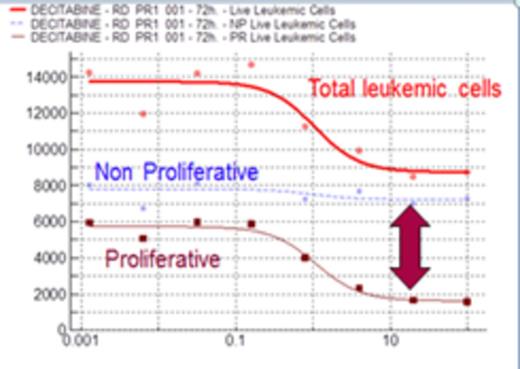
Results: To corroborate the hypothesis that AZA or DAC induce cell cycle arrest and this effect is different from cytotoxicity, we evaluated the effect of DAC in an AML cell line (KG-1) that grow spontaneously with medium and serum. As shown in figure 1, a decrease in the number of cells is observed when the AML cell line (KG-1) is incubated with DAC (blue line). Interestingly, an exactly proportional increase in the Mean Fluorescent Intensity (MFI) of CFDA is observed (red line), the symmetry between these curves indicate they measure the same effect; the drug-concentration dependent decrease in proliferating cells. Green line represents the percentage of apoptosis, as a measurement of the cytotoxic activity, clearly different that the other 2 measurements and only relevant at much higher doses, indicating that DAC has an essentially antiproliferative effect in the cell line. Once tested the hypothesis, we translate the idea to fresh AML samples inducing the blast proliferation with a very complete cytokine medium. In all patients included in this study, an average population of 47.2%±16.9% proliferating cells was identified. A clear difference in the sensitivity of no proliferative vs proliferative cell subset was observed, while DAC had no activity on the no proliferating cells (flat dose response in Figure 2), it was potently (1 uM) inhibiting almost completely proliferation in the proliferating cell subset.
Conclusion: Although the mechanism of action of hypomethylating agents is not still entirely clear, we hypothesize that is associated with antiproliferative effects. In this study, using our Exvitech platform we have developed an ex-vivo model to measure the antiproliferative effect of hypomethylating agents, using whole stimulated bone marrow samples from AML patients. If this new assay would correlate with patient treatment response, it could become a valuable tool to personalize hypomethylating treatments for AML patients.
Hernandez:Vivia Biotech: Employment. Gomez:Vivia Biotech: Employment. Robles:Vivia Biotech: Employment. Gorrochategui:Vivia Biotech: Employment. Sanchez:Vivia Biotech: Employment. Ballesteros:Vivia Biotech: Employment. Martinez:Vivia Biotech: Membership on an entity's Board of Directors or advisory committees.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal