Abstract
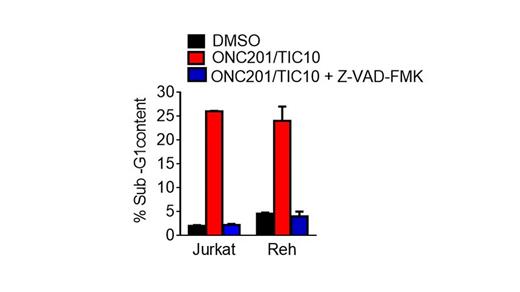
ONC201/TIC10 is a potent small molecule anti-tumor agent in several types of solid tumors and lymphomas. ONC201/TIC10 is on track to enter clinical trials for patients with advanced cancer in 2014, with IND issued by the FDA in March, 2014. Early trials will evaluate the safety and efficacy of ONC201/TIC10 as a monoagent in hematological malignancies. In the current study, we evaluated the anti-cancer effects of the small molecule in Acute Lymphoblastic Leukemia (ALL). Analysis of cell viability by the CellTiter-Glo method revealed that ONC201/TIC10 treatment reduces the viability of three ALL cell lines (Reh, Jurkat, MOLT-4) in a dose- (2.5/5/10 μM) and time-dependent manner (24/48/72 h). We have previously reported that ONC201/TIC10-mediated reduction in cell viability and apoptosis in various types of solid tumors occurs at 60/72 h. Interestingly, ONC201/TIC10 reduces the viability of ALL cell lines within 24/48 h at the indicated doses. An inactive TIC10 isomer compound synthesized by Medkoo Biosciences with a structure related to the active ONC201/TIC10 compound does not reduce the viability of ALL cells. Sub-G1 analysis indicated that ONC201/TIC10 induces apoptosis in ALL cells and a pan-caspase inhibitor reduces ONC201/TIC10-mediated apoptosis. Western blot analysis was used to further investigate the mechanism of ONC201/TIC10-mediated apoptosis. ONC201/TIC10-mediated apoptosis involves PARP cleavage and caspase-9 activation. Anti-apoptotic Bcl-2 family members Bcl-2 and Bcl-xl are downregulated while the pro-apoptotic Bcl-2 family member Bim is upregulated in response to ONC201/TIC10 treatment. ONC201/TIC10 also downregulates the inhibitor of apoptosis (IAP) family proteins cIAP1 and cIAP2. We have previously shown that the anti-tumor effect of ONC201/TIC10 involves inhibition Akt and ERK phosphorylation resulting in Foxo3a activation and TRAIL-gene transcription. We observed inhibition of Akt phosphorylation upon ONC201/TIC10 treatment of ALL cells. Thus, ONC201/TIC10 holds promise as a novel agent for the treatment of ALL based on its robust activity in preclinical models of the disease. Our ongoing studies are evaluating the impact of this novel therapy on ALL cells with different translocations, and are introducing combination therapy with ONC201/TIC10 for ALL.
Allen:Oncoceutics: Employment, Equity Ownership, Patents & Royalties. El-Deiry:Oncoceutics, Inc.: Equity Ownership, Patents & Royalties.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal