Abstract
Background: Ponatinib is an approved potent oral tyrosine kinase inhibitor active against native and mutated forms of BCR-ABL, including T315I. The phase 2 PACE study demonstrated that ponatinib is highly active in heavily pretreated Philadelphia chromosome‒positive leukemia patients. Ponatinib efficacy and safety were evaluated in newly diagnosed CP-CML patients in the EPIC trial.
Methods: EPIC was a multicenter, international, phase 3, randomized, 2-arm, open-label trial of ponatinib (45 mg once daily) compared with imatinib (400 mg once daily) in newly diagnosed CP-CML; patients were stratified by Sokal risk score (low [<0.8] vs intermediate [0.8 to ≤1.2] vs high [>1.2]). On 18 October 2013,EPIC was terminated due to the observation of arterial thrombotic events in the ponatinib development program. Consequently, none of the prospectively defined endpoints could be analyzed. Data as of 1 April 2014 are presented for endpoints that could be analyzed: BCR-ABLIS <10% rate at 3 months; major molecular response (MMR), molecular response (MR)4, and MR4.5 rates at and after at least 3, 6, 9, and 12 months and at any time; time to response; complete cytogenetic response rates at 6 and 12 months and any time; and safety.
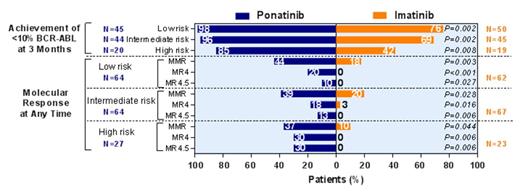
Results: At the time of study termination, 307 patients had been randomized; median follow-up was 5.1 (0.03-17.6) months. Groups were well-balanced with respect to sex, age, pretreatment, and Sokal score; however, the proportion of patients with 1 or more cardiovascular risk factors (hypertension, hypercholesterolaemia, diabetes, obesity and smoking) was higher in the ponatinib arm (n=97, 63%) compared to the imatinib arm (n=79, 52%). Data were available on 306 treated patients (154 ponatinib, 152 imatinib). Fourteen ponatinib and 2 imatinib patients discontinued due to adverse events (AEs). Molecular response rates for ponatinib were uniformly higher compared with imatinib for all response measures and at all time points (Table). The percentage of patients who achieved <10% BCR-ABL at 3 months was significantly higher in the ponatinib compared with imatinib arm overall (Table), and when patients were stratified by high-risk, intermediate-risk, and low-risk Sokal score (Figure). The percentage of patients who achieved MMR, MR4, and MR4.5 at any time in all Sokal risk groups was higher for ponatinib than imatinib (Figure). The most common (≥25%) all-grade treatment-emergent AEs with ponatinib were rash (38%), abdominal pain (36%), headache (33%), constipation (27%), increased lipase (27%), myalgia (26%), and thrombocytopenia (25%); with imatinib, they were nausea (34%), muscle spasms (34%), and diarrhea (27%). Twelve percent of ponatinib and 7% of imatinib patients had grade 3/4 thrombocytopenia; 3% of ponatinib and 8% of imatinib patients had grade 3/4 neutropenia. Serious treatment-emergent AEs (SAEs) occurring in ≥3 ponatinib patients were pancreatitis (n=5), atrial fibrillation (n=3), and thrombocytopenia (n=3); no individual SAEs occurred in ≥3 imatinib patients. Eleven (7%) ponatinib and 3 (2%) imatinib patients experienced arterial thrombotic events, designated serious for 10 [7%] ponatinib and 1 [0.7%] imatinib patient(s). One patient in the ponatinib arm experienced a serious venous thromboembolic event: there were none in the imatinib arm. Ten of 11 ponatinib patients, and 2 of 3 imatinib patients with arterial thrombotic events had 1 or more cardiovascular risk factors.
Conclusions: Despite early termination, at a median follow-up of 5 months, preliminary evidence suggests that ponatinib has improved efficacy over imatinib in newly diagnosed CP-CML patients, but has a higher AE rate, including ATEs at the dose studied. Future investigations of ponatinib in the frontline setting will likely use lower doses and account for relevant risk factors.
| Table: Molecular Response Rates | ||||||||||
| At 3 months | At 6 months | At 9 months | At 12 months | At any time | ||||||
| Ponatinib N=109 | Imatinib N=114 | Ponatinib N=69 | Imatinib N=73 | Ponatinib N=22 | Imatinib N=27 | Ponatinib N=10 | Imatinib N=13 | Ponatinib N=149 | Imatinib N=142 | |
| MMR, n (%) | 34 (31) | 3 (3) | 43 (62) | 16 (22) | 19 (86) | 9 (33) | 8 (80) | 5 (39) | 61 (41) | 25 (18) |
| MR4, n (%) | 8 (7) | 0 | 22 (32) | 1 (1) | 14 (64) | 1 (4) | 6 (60) | 0 | 31 (21) | 2 (1) |
| MR4.5, n (%) | 5 (5) | 0 | 11 (16) | 0 | 7 (32) | 0 | 6 (60) | 0 | 22 (15) | 0 |
| ≤10% BCR-ABL transcripts, n (%) | 103 (94) | 77 (68) | ||||||||
| Table: Molecular Response Rates | ||||||||||
| At 3 months | At 6 months | At 9 months | At 12 months | At any time | ||||||
| Ponatinib N=109 | Imatinib N=114 | Ponatinib N=69 | Imatinib N=73 | Ponatinib N=22 | Imatinib N=27 | Ponatinib N=10 | Imatinib N=13 | Ponatinib N=149 | Imatinib N=142 | |
| MMR, n (%) | 34 (31) | 3 (3) | 43 (62) | 16 (22) | 19 (86) | 9 (33) | 8 (80) | 5 (39) | 61 (41) | 25 (18) |
| MR4, n (%) | 8 (7) | 0 | 22 (32) | 1 (1) | 14 (64) | 1 (4) | 6 (60) | 0 | 31 (21) | 2 (1) |
| MR4.5, n (%) | 5 (5) | 0 | 11 (16) | 0 | 7 (32) | 0 | 6 (60) | 0 | 22 (15) | 0 |
| ≤10% BCR-ABL transcripts, n (%) | 103 (94) | 77 (68) | ||||||||
Patients Achieving <10% BCR-ABL Transcript Levels at 3 Months and Molecular Response (MMR, MR4, MR4.5) at Any Time, by Sokal Risk Score
Patients Achieving <10% BCR-ABL Transcript Levels at 3 Months and Molecular Response (MMR, MR4, MR4.5) at Any Time, by Sokal Risk Score
Lipton:Novartis, BMS, Pfizer: Honoraria, Research Funding, Speakers Bureau; Novartis, BMS, Pfizer, Teva: Consultancy. Off Label Use: Ponatinib is a BCR-ABL kinase inhibitor that has been approved by the US FDA for the treatment of adult patients with CML (all phases) or Ph+ ALL that is T315I-positive or for whom no other TKI therapy is indicated.. Chuah:Novartis, BMS: Honoraria. Assouline:Pfizer, Novartis: Honoraria, Research Funding. Etienne:Novartis, BMS,Pfizer, ARIAD Pharmaceuticals, Inc.: Consultancy, Membership on an entity's Board of Directors or advisory committees, Research Funding. Nicolini:Novartis, BMS, ARIAD Pharmaceuticals, Inc.: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding. Le Coutre:ARIAD Pharmaceuticals, Inc., Novartis, BMS, Pfizer: Honoraria. Clark:Novartis, Sanofi Aventis: Speakers Bureau; Novartis, Pfizer, Sanofi Aventis: Honoraria; Novartis, BMS, Pfizer, Sanofi Aventis: Research Funding. Stenke:ARIAD Pharmaceuticals, Inc.: Membership on an entity's Board of Directors or advisory committees. Oehler:ARIAD Pharmaceuticals, Inc.: Advisory board Other. Lustgarten:ARIAD Pharmaceuticals, Inc.: Employment, Equity Ownership. Rivera:ARIAD Pharmaceuticals, Inc.: Employment, Equity Ownership. Clackson:ARIAD Pharmaceuticals, Inc.: Employment, Equity Ownership. Haluska:ARIAD Pharmaceuticals, Inc.: Employment, Equity Ownership. Baccarani:ARIAD, Novartis, BMS, Pfizer, Teva: Honoraria, Speakers Bureau; ARIAD, Novartis, BMS: Consultancy. Cortes:ARIAD, BMS, Novartis, Pfizer, Teva: Consultancy, Research Funding. Guilhot:ARIAD Pharmaceuticals, Inc.: Honoraria. Hochhaus:ARIAD Pharmaceuticals, Inc.: Research Funding. Hughes:Novartis, BMS, ARIAD: Honoraria, Research Funding. Kantarjian:ARIAD, Pfizer, Amgen: Research Funding. Shah:ARIAD Pharmaceuticals, Inc., BMS: Research Funding. Talpaz:ARIAD Pharmaceuticals, Inc., BMS, Sanofi, Incyte, Pfizer: Research Funding. Deininger:BMS, ARIAD, Novartis, Incyte, Pfizer: Consultancy; BMA, ARIAD, Novartis, Incyte, Pfizer: Advisory Board, Advisory Board Other; BMS, Novartis, Celgene, Genzyme, Gilead: Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal