Abstract
Novel chronic lymphocytic leukemia (CLL) therapies target phosphoinositide 3-kinase (PI3K; idelalisib) and Bruton's tyrosine kinase (BTK; ibrutinib). Despite their success, certain high-risk groups, notably those with dysfunctional P53, demonstrate lower response rates than other CLL groups. As such, combination therapies could potentially overcome this deficiency. As CD37-mediated signaling resulted in direct cytotoxicity that was enhanced with PI3K inhibition (Lapalombella 2012), we aimed to combine BI 836826, a novel IgG1 chimerized and Fc-engineered anti-CD37 mAb, with BCR-pathway inhibitors to possibly enhance clinical efficacy in high-risk CLL patients and overcome potential inhibition of ADCC.
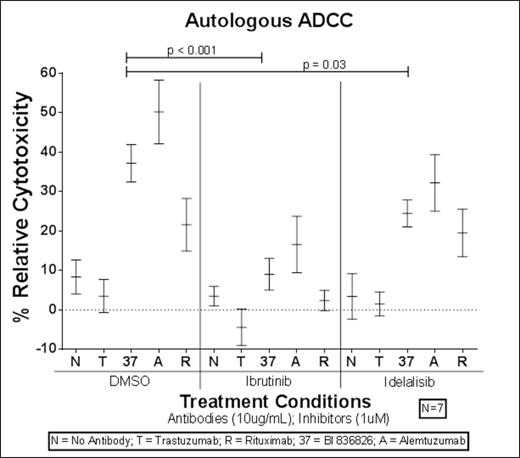
Consistent with previous report, BI 836826 displays both ADCC and direct pro-apoptotic activity (Heider 2011). We assessed the potential effect of idelalisib and ibrutinib on CD37 expression and NK-cell function. Following 24hr incubation of primary CLL cells (n=4) with idelalisib, ibrutinib, or DMSO, there was no significant difference in surface expression of CD37 (fold change ~ 1.01; p > 0.16 for all comparisons). To ensure that NK-cell Fc-receptor function was unimpaired by BCR-pathway inhibitors, we conducted CD107ab degranulation assays (n=5). Data revealed that Fc-receptor engagement of BI 836826 and NK-cell lytic degranulation was not abrogated by idelalisib or ibrutinib (mean CD107ab expression: DMSO = 10.14%; idelalisib = 5.67%; ibrutinib = 2.41%). In combination with idelalisib, BI 836826 was still capable of eliciting an effective NK-cell response superior to rituximab (difference = 4.51%; p < 0.0001). To further qualify NK-cell function, TNFα (n = 6) or INFγ (n = 12) release by NK cells after pretreatment with idelalisib or ibrutinib was evaluated. Similarly, cytokine release was not completely inhibited by idelalisib and ibrutinib; and BI 836826 was still capable of inducing cytokine release. To confirm the potential added benefit of combination therapy, we conducted 51Cr release ADCC assays with primary CLL cells and healthy allogeneic NK-cells (Fig1) or CLL patient autologous NK-cells (Fig2). Results confirmed that neither ibrutinib nor idelalisib could ablate BI 836826-induced NK-cell ADCC. However, there was a more pronounced inhibition of NK-cell ADCC with ibrutinib. As previously demonstrated (Kohrt 2014), NK-cell ADCC mediated by rituximab was almost completely abrogated by ibrutinib, likely secondary to off-target interleukin-2-inducible T-cell kinase inhibition (Dubovsky 2013).
Our preliminary data show that signaling induced by BI 836826 may demonstrate the same enhanced direct cytotoxicity with PI3K inhibition as previously reported with other CD37 mediated signaling agents (Lapalombella 2012). Evaluation of direct pro-apoptotic activity of BI 836826 (0.1ug/mL) in combination with idelalisib (1uM) on direct cell death (24hr incubation) demonstrated enhanced activity in the combination setting with similar activity in primary patient CLL cells with dysfunctional P53 (n=6; Fig3A) and functional P53 (n=4; Fig3B).
In conclusion, these results demonstrate that idelalisib could potentially be used in combination with BI 836826, which has the added benefit of directly killing inhibitor-resistant P53-null primary CLL cells and lacks complete inhibitor-induced ADCC ablation.
Allogeneic ADCC after Pretreatment of NK-cells with BTK inhibitor (Ibrutinib) or PI3K inhibitor (Idelalisib)
Allogeneic ADCC after Pretreatment of NK-cells with BTK inhibitor (Ibrutinib) or PI3K inhibitor (Idelalisib)
Autologous ADCC after Pretreatment of NK-cells with BTK inhibitor (Ibrutinib) or PI3K inhibitor (Idelalisib)
Autologous ADCC after Pretreatment of NK-cells with BTK inhibitor (Ibrutinib) or PI3K inhibitor (Idelalisib)
Off Label Use: Off-label use of idelalisib and BI 836826 in combination for CLL patients. Jones:Pharmacyclics: Consultancy, Research Funding. Heider:Boehringer Ingelheim: Employment.
Author notes
Asterisk with author names denotes non-ASH members.



This feature is available to Subscribers Only
Sign In or Create an Account Close Modal