Abstract
Background: CC-486 is an oral formulation of the epigenetic modifier, azacitidine, in clinical development for treatment (Tx) of hematological cancers. Part 1 of this phase I, multicenter study showed no effect on pharmacokinetics (PK) of CC-486 when taken with food or with a proton-pump inhibitor (Laille, 2014). A prospective extension phase of this study is ongoing to assess the safety and efficacy of CC-486.
Objectives: To assess safety, tolerability, and hematological response associated with an extended CC-486 dosing schedule in patients (pts) with MDS, AML, or CMML.
Methods: Eligible pts were age ≥18 years, had confirmed WHO-defined MDS, CMML, or AML based on bone marrow (BM) aspirate and biopsy, and an ECOG performance status score 0-2. In the PK phase, pts received 2 to 3 single CC-486 doses and in the extension phase, pts received CC-486 300 mg QD for the first 21 days of repeated 28-day cycles. Tx-emergent adverse events (TEAEs) were coded by MedDRA v15.0 and graded using NCI-CTCAE v4.0. In addition to TEAEs for all pts, because PK-phase exposure was quite limited, TEAEs of interest are reported for the subset of pts who entered the extension phase (21-day dosing). Responses (complete or partial remission [CR, PR], CR with incomplete blood count recovery [CRi], marrow CR [mCR], hematologic improvement [HI], and transfusion independence [TI]) were defined by IWG 2006 (MDS) or IWG 2003 (AML) criteria. Baseline transfusion dependence (TD) was defined as ≥4 red blood cell (RBC) units or ≥2 platelet transfusions, in the 56 days before first dose. TI was defined as no transfusion for 56 consecutive days. The safety-evaluable cohort included all pts who received ≥1 CC-486 dose. Efficacy-evaluable pts had ≥1 post-baseline efficacy measurement during the extension phase as of data cut-off (June 6, 2014). Efficacy results are reported for the combined MDS and CMML pt population and separately for pts with AML.
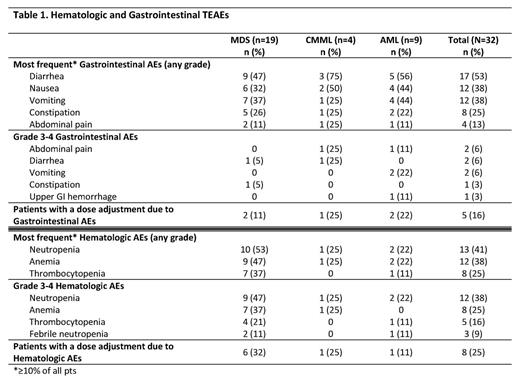
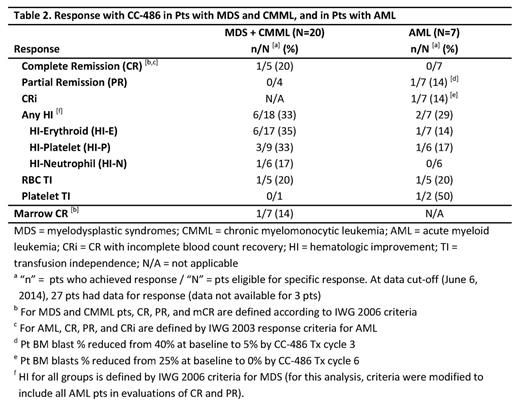
Results: A total of 32 pts (MDS n=19 [59%], CMML n=4 [13%], and AML n=9 [28%]) received ≥1 CC-486 dose and are evaluated for safety. Median (range) age of all pts was 72 (53-93) years, with 44% of pts age ≥75 years. Most pts were male (69%) and all were Caucasian. Median number of CC-486 Tx cycles for all pts was 4 (range 1-18), and within the MDS, CMML, and AML groups was 5 (1-18), 4 (2-17), and 1 (1-9), respectively. The mean ±SD Tx cycle length was 30 ±9.0 days overall (by group: 33 ±8.3 days in MDS, 25 ±5.4 in CMML, and 27 ±10.7 in AML pts). The most frequent types of TEAE were gastrointestinal (GI) and hematological (Table 1). CC-486 dose was adjusted due to GI TEAEs for 5 pts (16%), and due to hematological TEAEs for 8 pts (25%). Of all 32 pts, 30 pts (94%) entered the 21-day dosing extension phase. Of these pts, GI TEAEs (grade ≥2) were reported for 23 pts (77%). Of these, only 4 grade 3 (vomiting and diarrhea, 2 each) and no grade 4 TEAEs were observed. Hematological TEAEs (grade ≥3) were reported for 19 pts (63%). Neutropenic grade ≥2 TEAEs occurred in 12 (40%) of 30 pts, of which 29% were grade 2, 38% grade 3, and 32% grade 4. Febrile neutropenia occurred in 3 pts and grade 4 thrombocytopenia in 3. Most TEAEs occurred during the first 2 Tx cycles: GI, 73% and hematological, 55%. The efficacy-evaluable population at data cut-off included 27 pts (84%): 20 pts with MDS or CMML, and 7 pts with AML (Table 2). One pt with MDS achieved CR, 1 pt with AML achieved CRi, and 1 pt with AML achieved PR (pt was to proceed to transplant). Of HI-evaluable pts in the MDS+CMML and AML groups, respectively, 6/18 (33%) and 2/7 (29%) attained an HI response. Few pts were TD at baseline. Of pts who were RBC TD at baseline, 1/5 pts (20%) in the MDS+CMML group and 1/5 pts (20%) in the AML group attained RBC TI. Of 3 pts who were platelet TD at baseline, 1 pt with AML attained platelet TI.
Conclusions: CC-486 can provide the flexibility to adjust dose or dosing regimen to improve tolerability or efficacy as needed. The safety and tolerability of CC-486 300 mg QD taken for the first 21 days of repeated 28-day cycles were consistent with the known safety profile of injectable azacitidine in these pts with MDS, CMML, and AML. Grade 3-4 gastrointestinal TEAEs were infrequent (<20% of all pts). Grade 3-4 neutropenias were observed and deserve scrutiny, especially in the first 2 Tx cycles, and in some cases may warrant initiation of prophylactic antibiotics, particularly in pts with prolonged grade 4 neutropenia. HI rates of ~30% observed in this trial were similar to previous reports of CC-486.
Savona:Karyopharm: Consultancy, Equity Ownership; Celgene: Consultancy; Gilead: Consultancy; Incyte: Consultancy. Kolibaba:Celgene: Research Funding. Conkling:Celgene: Research Funding. Rifkin:Celgene: Scientific Advisory Board Other; Millennium/Takeda: Scientific Advisory Board, Scientific Advisory Board Other; Onyx/Amgen: Scientific Advisory Board, Scientific Advisory Board Other. Laille:Celgene: Employment, Equity Ownership. Kellerman:Celgene: Employment. Ukrainskyj:Celgene: Employment, Equity Ownership. Dong:Celgene: Employment. Skikne:Celgene: Employment, Equity Ownership.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal