Abstract
Current chemotherapeutic approaches in AML and MDS target rapidly dividing cells, having limited effects on the leukemic and preleukemic stem cells responsible for disease propagation and relapse. Novel therapies that are able to target (pre)leukemic stem cells are urgently needed to maintain remission and improve survival in AML and MDS. We recently identified the putative transcription factor H2.0-like homeobox (HLX) to be upregulated in pre-leukemic stem cells and in 87% of patients with AML, and showed that HLX is functionally critical in AML pathogenesis (Cancer Cell 2012; 22(2):194-208). As transcription factors are still challenging clinical targets, we sought to identify key downstream molecules with available small molecule inhibitors that mediate the leukemia promoting effects of HLX. We identified p21-activated kinase (PAK1) and found that high expression of PAK1 is associated with significantly inferior overall survival in AML and MDS. Assessing the functional importance of PAK1 in AML and MDS, we found that inhibition of PAK1 activity by small molecule inhibitors (IPA-3, an allosteric inhibitor of PAK1-3 activation, and FRAX-597, an ATP-competitive PAK1-3 inhibitor) leads to profound growth-inhibitory effects and induction of differentiation and apoptosis of AML cell lines (THP-1, MOLM-13, HL-60, KG1a) in vitro at nM to low uM dosages. Likewise, PAK1 inhibition by shRNA-mediated knockdown led to significant inhibition of cell proliferation and clonogenicity, induction of apoptosis, and differentiation of AML cells. In vivo xenotransplantation of THP-1 cells infected with non-silencing control or PAK1 knockdown lentiviruses into NSG mice showed a striking 91% reduction in infiltration of leukemic cells, and preservation of normal tissue architecture in the bone marrow, spleen, and liver upon PAK1 knockdown. At the molecular level, inhibition of PAK1 in THP-1 cells by either small molecule led to the loss of AKT activity and revealed downregulation of MYC and a core network of MYC target genes.
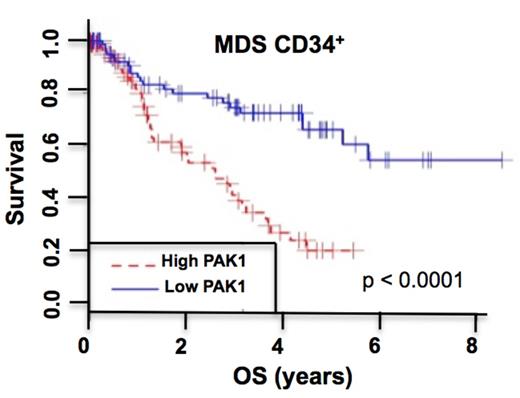
As MDS are pre-leukemic conditions showing frequent progression to AML we assessed PAK1 expression in the stem cell-enriched (CD34+) cell population from 183 patients with MDS. We found that MDS progression is associated with increasing levels of PAK1 in MDS CD34+ cells, and that high PAK1 expression correlates with inferior overall survival across all MDS subtypes (Figure 1; and data not shown). These data led us to investigate whether leukemic and/or normal stem and progenitor cells are dependent on PAK1 function. Inhibition of PAK1 activity by IPA-3 or FRAX-597 led to a highly significant, dose-dependent reduction of malignant colony formation of primary AML patients' cells, while colony-forming capacity of healthy control cells was only modestly affected. Finally, we assessed the effectiveness of PAK1 inhibition specifically at the level of CD34+CD38- leukemic stem cells. Strikingly, PAK1 inhibition had a profound inhibitory effect on leukemic CD34+CD38- cells while sparing healthy CD34+CD38- stem cells. In summary, our studies identify PAK1 as a novel therapeutic target in AML and MDS, provide cell biological and molecular insights into PAK1 function in myeloid malignancies, and provide a preclinical rationale for the testing of PAK1 inhibitors as a therapeutic strategy in AML and MDS.
Kaplan-Meier curve of overall survival of patients with high versus low PAK1 expression in CD34+ bone marrow cells isolated from 183 MDS patients (GSE19429). Patients with high PAK1 expression are those with PAK1 expression levels higher than the median expression level of PAK1 in the dataset. p value (log-rank test) are indicated.
Kaplan-Meier curve of overall survival of patients with high versus low PAK1 expression in CD34+ bone marrow cells isolated from 183 MDS patients (GSE19429). Patients with high PAK1 expression are those with PAK1 expression levels higher than the median expression level of PAK1 in the dataset. p value (log-rank test) are indicated.
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal