Abstract
Background:
Total lymphocyte count (TLC) has been shown to correlate with outcomes in patients (pts) with acute leukemia. The clinical correlation to TLC in pts with chronic myeloid leukemia in chronic phase (CML-CP) who were treated with a tyrosine-kinase inhibitor (TKI) is unclear.
Methods:
Lymphocyte data in pts with newly diagnosed CML-CP who were enrolled in consecutive or parallel clinical trials with front-line imatinib (IM), nilotinib (Nilo), or dasatinib (Dasa) were collected at the time of diagnosis, and 3 and 6 months (M) after the start of TKI. Relative lymphocytrosis (RLC) was defined as lymphocyte >150% at 3 or 6M compared with baseline at diagnosis. Absolute lymphocytosis (ALC) was defined as lymphocyte > 4,000 /µL at 3 or 6M after the start of TKI. Pts were assessed for response, overall survival (OS), event-free survival (EFS), transformation-free survival (TFS), and failure-free survival (FFS) based on ALC and RLC. The Kaplan-Meier method was used to calculate OS, EFS, TFS, and FFS. A log-rank test and Cox regression were used for univariate (UVA) and multivariate analysis (MVA), respectively.
Results:
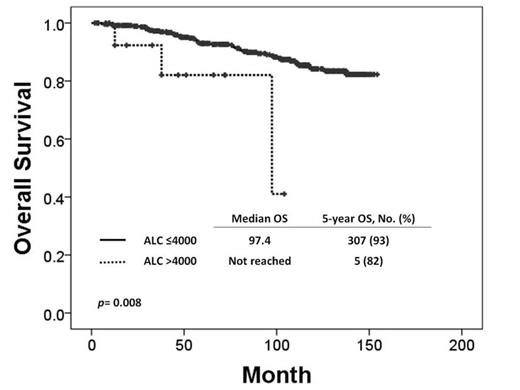
A total of 483 pts were enrolled in this study: 271 in IM, 105 in Nilo, and 107 in Dasa. Patient characteristics and outcomes are summarized in Table 1. Median age at diagnosis was 48 years, and median follow-up was 85M and ongoing (5-154+). Time from diagnosis to start of TKI, Sokal risk score, and ALC at baseline between groups did not differ clinically. Of 481 pts, 93 (19%) developed RLC at 3 or 6M; IM, 38 (14%); Nilo, 23 (22%); Dasa, 32 (30%) (p= .001). ALC at 3 or 6M was observed in 15 (3%); IM, 3 (1%); Nilo, 1 (1%); Dasa, 11 (10%) (p<.001). Overall, cumulative incidence of complete cytogenetic response (CCyR) at 6M, major molecular response (MMR) at 12M, molecular response with 4.5 log reduction by IS (MR4.5) at 24M did not differ significantly between RLC and non-RLC (3 or 6M), or between ALC and non-ALC (3 or 6M). 5-y TFS, EFS and OS in ALC group were significantly worse than those in non-ALC group (p= .002, p=.016, p=.008, respectively). By UVA and MVA related to OS, age [p <.001; Hazard ratio (HR), 1.062; 95% confidence interval (95%CI), 1.036-1.089], presence of ALC at 3 or 6M [p = .028; HR, 10.948; 95%CI, 1.297-92.415], absence of MMR at 24M [p=.016; HR, 2.263; 95%CI, 1.165-4.393] were identified as adverse prognostic factors for OS.
Conclusion:
The presence of ALC ≥4,000/µL at 3 or 6M of TKI therapies is rare but is adversely associated with overall survival.
Patient Characteristics and Outcomes (N=483)a
| . | Overall [n= 481] . | IM [n= 271] . | Nilo [n= 105] . | Dasa [n= 107] . |
|---|---|---|---|---|
| Age, (year) | 48 (15-85) | 48 (15-85) | 49 (17-82) | 48 (16-83) |
| Sokal Risk, No. (%) | ||||
| Low | 334 (69) | 175 (65) | 79 (75) | 80 (75) |
| Intermediate | 114 (24) | 74 (27) | 18 (17) | 22 (21) |
| High | 32 (7) | 20 (7) | 8 (8) | 4 (4) |
| Time from diagnosis to start of TKI, (M) | 0.9 (0-12.6) | 1.0 (0-12.6) | 0.5 (0-5.6) | 0.7 (0.1-7.8) |
| ALC at baseline, (/109L) | 2.5 (0-86.6) | 2.4 (0-16.7) | 2.6 (0.4-9.2) | 2.7 (0.3-86.6) |
| Incidence of Relative Lymphocytosis, No. (%) | ||||
| At 3M | 65 (14) | 25 (9) | 16 (15) | 24 (22) |
| At 6M | 76 (16) | 32 (12) | 20 (19) | 24 (22) |
| Overall | 93 (19) | 38 (14) | 23 (22) | 32 (30) |
| Incidence of Absolute Lymphocytosis, No. (%) | ||||
| At 3M | 8 (2) | 1 (0) | 0 | 7 (7) |
| At 6M | 11 (2) | 3 (1) | 1 (1) | 7 (7) |
| Overall | 15 (3) | 3 (1) | 1 (1) | 11 (10) |
| Outcomes of RLC and ALC at any time in each group, +/- (%/%) (p) | ||||
| <10% BCR-ABL/ABL at 3M | ||||
| RLC | 36/40 (.596) | 22/44 (.213) | 50/37 (.280) | 31/38 (.537) |
| ALC | 38/39 (.952) | 0/42 (.394) | 100/39 (.214) | 36/35 (.952) |
| Cumulative CCyR at 6M | ||||
| RLC | 75/75 (.288) | 50/66 (.063) | 96/90 (.413) | 90/87 (.628) |
| ALC | 67/75 (.711) | 33/64 (.276) | 0/92 (.001) | 82/89 (.599) |
| Cumulative MMR at 12M | ||||
| RLC | 67/74 (.406) | 53/70 (.030) | 83/82 (.921) | 72/74 (.903) |
| ALC | 60/73 (.488) | 33/68 (.197) | 0/83 (.033) | 73/74 (.745) |
| Cumulative MR4.5 at 24M | ||||
| RLC | 46/52 (.564) | 37/50 (.139) | 57/55 (.889) | 50/57 (.729) |
| ALC | 33/52 (.332) | 33/48 (.610) | 0/56 (.264) | 36/57 (.252) |
| 5-y FFS | ||||
| RLC | 61/71 (.133) | 56/69 (.167) | 62/70 (.710) | 61/74 (.285) |
| ALC | 50/69 (.076) | 0/68 (<.001) | 0/70 (<.001) | 71/70 (.974) |
| 5-y TFS | ||||
| RLC | 90/93 (.369) | 88/93 (.597) | 91/88 (.115) | 91/99 (.213) |
| ALC | 72/93 (.002) | 67/93 (.014) | 0/90 (<.001) | 80/97 (.121) |
| 5-y EFS | ||||
| RLC | 80/86 (.213) | 71/83 (.154) | 84/87 (.450) | 86/93 (.486) |
| ALC | 64/85 (.016) | 33/82 (<.001) | 0/87 (<.001) | 80/92 (.574) |
| 5-y OS | ||||
| RLC | 89/93 (.068) | 81/94 (.007) | 100/84 (.126) | 96/99 (.207) |
| ALC | 82/93 (.008) | 67/93 (.001) | 100/88 (.847) | 83/99 (.040) |
| . | Overall [n= 481] . | IM [n= 271] . | Nilo [n= 105] . | Dasa [n= 107] . |
|---|---|---|---|---|
| Age, (year) | 48 (15-85) | 48 (15-85) | 49 (17-82) | 48 (16-83) |
| Sokal Risk, No. (%) | ||||
| Low | 334 (69) | 175 (65) | 79 (75) | 80 (75) |
| Intermediate | 114 (24) | 74 (27) | 18 (17) | 22 (21) |
| High | 32 (7) | 20 (7) | 8 (8) | 4 (4) |
| Time from diagnosis to start of TKI, (M) | 0.9 (0-12.6) | 1.0 (0-12.6) | 0.5 (0-5.6) | 0.7 (0.1-7.8) |
| ALC at baseline, (/109L) | 2.5 (0-86.6) | 2.4 (0-16.7) | 2.6 (0.4-9.2) | 2.7 (0.3-86.6) |
| Incidence of Relative Lymphocytosis, No. (%) | ||||
| At 3M | 65 (14) | 25 (9) | 16 (15) | 24 (22) |
| At 6M | 76 (16) | 32 (12) | 20 (19) | 24 (22) |
| Overall | 93 (19) | 38 (14) | 23 (22) | 32 (30) |
| Incidence of Absolute Lymphocytosis, No. (%) | ||||
| At 3M | 8 (2) | 1 (0) | 0 | 7 (7) |
| At 6M | 11 (2) | 3 (1) | 1 (1) | 7 (7) |
| Overall | 15 (3) | 3 (1) | 1 (1) | 11 (10) |
| Outcomes of RLC and ALC at any time in each group, +/- (%/%) (p) | ||||
| <10% BCR-ABL/ABL at 3M | ||||
| RLC | 36/40 (.596) | 22/44 (.213) | 50/37 (.280) | 31/38 (.537) |
| ALC | 38/39 (.952) | 0/42 (.394) | 100/39 (.214) | 36/35 (.952) |
| Cumulative CCyR at 6M | ||||
| RLC | 75/75 (.288) | 50/66 (.063) | 96/90 (.413) | 90/87 (.628) |
| ALC | 67/75 (.711) | 33/64 (.276) | 0/92 (.001) | 82/89 (.599) |
| Cumulative MMR at 12M | ||||
| RLC | 67/74 (.406) | 53/70 (.030) | 83/82 (.921) | 72/74 (.903) |
| ALC | 60/73 (.488) | 33/68 (.197) | 0/83 (.033) | 73/74 (.745) |
| Cumulative MR4.5 at 24M | ||||
| RLC | 46/52 (.564) | 37/50 (.139) | 57/55 (.889) | 50/57 (.729) |
| ALC | 33/52 (.332) | 33/48 (.610) | 0/56 (.264) | 36/57 (.252) |
| 5-y FFS | ||||
| RLC | 61/71 (.133) | 56/69 (.167) | 62/70 (.710) | 61/74 (.285) |
| ALC | 50/69 (.076) | 0/68 (<.001) | 0/70 (<.001) | 71/70 (.974) |
| 5-y TFS | ||||
| RLC | 90/93 (.369) | 88/93 (.597) | 91/88 (.115) | 91/99 (.213) |
| ALC | 72/93 (.002) | 67/93 (.014) | 0/90 (<.001) | 80/97 (.121) |
| 5-y EFS | ||||
| RLC | 80/86 (.213) | 71/83 (.154) | 84/87 (.450) | 86/93 (.486) |
| ALC | 64/85 (.016) | 33/82 (<.001) | 0/87 (<.001) | 80/92 (.574) |
| 5-y OS | ||||
| RLC | 89/93 (.068) | 81/94 (.007) | 100/84 (.126) | 96/99 (.207) |
| ALC | 82/93 (.008) | 67/93 (.001) | 100/88 (.847) | 83/99 (.040) |
a Two in IM and 1 in Dasa were not evaluable due to lack of differential data at 3 and 6M.
O'Brien:Amgen, Celgene, GSK: Consultancy; CLL Global Research Foundation: Membership on an entity's Board of Directors or advisory committees; Emergent, Genentech, Gilead, Infinity, Pharmacyclics, Spectrum: Consultancy, Research Funding; MorphoSys, Acerta, TG Therapeutics: Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.
This icon denotes a clinically relevant abstract

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal