Abstract
Introduction: Immune suppression is a major component of lymphoma pathogenesis. Recent advances in lymphoma treatment have been immunotherapy. We present the preliminary results of our clinical trial testing 2 vaccine strategies in patients with indolent B-cell non-Hodgkin lymphoma (NHL). The primary objective is to determine the safety and feasibility of the vaccine approaches and secondary objectives are to describe clinical responses and identify corresponding immune changes.
Methods: Autologous mature dendritic cells (mDC) were manufactured from leukapheresed cells of NHL pts. For patients with tumor lymph nodes deemed amenable to cryoablation by interventional radiologist (arm A), they received cryoablation of a node and injection of mDC into the cryoablated node followed by another 1 to 7 intratumoral mDC injections. Remaining patients had a tumor excised to generate tumor lysate ex vivo. mDC were pulsed with tumor lysate during DC maturation (arm B; DC-TL). The DC-TL vaccines were injected intradermally for 4 to 8 doses. Patients are monitored for one year after vaccines for adverse events and systemic tumor response. Correlative studies include cellular immune phenotype of peripheral blood and T cell intracellular cytokine productions. Planned accrual is 10 patients per arm (total = 20).
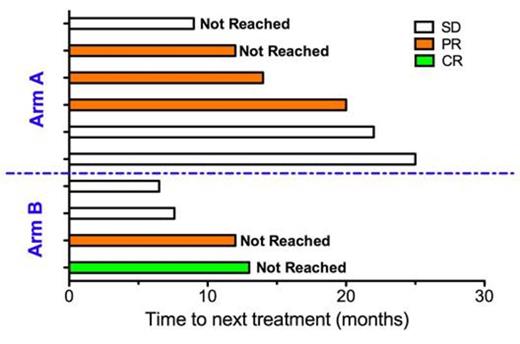
Results: To date, 10 patients have accrued to arm A and 5 patients to arm B (Table 1). All pts tolerated vaccine treatments without major adverse events. Of the 10 evaluable patients, there were 1 CR (arm B: 1 / 4; total: 1/6) and 4 PR (arm A: 3/6; arm B: 1 / 4; total: 4/10) for an ORR of 50% for both arms (Figure 1). Time to next treatment ranged from 6.5 to 25 months (Figure 2). Correlative studies suggest that immune changes can be used as prognostic biomarkers to predict response. These include changes in vaccine induced cytokine secretion, and in the number and composition of the immune system. In addition, preliminary analysis of >40 immune phenotypes using flow cytometry and hierarchical clustering suggest there are characteristic immune system responses in patients responding to vaccine.
Conclusions: Both cryoablation and intratumoral mDC vaccination are feasible and safe in NHL. Treatment responses may correlate with immune system changes. Biosystems analysis method can be used to develop novel assays as predictive biomarkers of treatment response and used to develop novel protocols that allow treating to response.
Patient Demographics
| . | Arm A (n = 10) . | Arm B (n = 5) . | Total (n = 15) . |
|---|---|---|---|
| Age (median, rage) | 64 (55 – 80) | 60 (31 – 86) | 63 (31 – 86) |
| Gender (M/F) | 6/4 | 2/3 | 8/7 |
| Histology (n, %) Follicular Marginal zone DLBCL | 10 (100%) 0 (0%) 0 (0%) | 3 (60%) 1 (20%) 1(20%) | 13 (86.6%) 1 (6.7%) 1 (6.7%) |
| Stage (N, %) II III IV | 1 (10%) 1 (10%) 8 (80%) | 2 (40%) 1 (20%) 2 (40%) | 3 (20%) 2 (13.3%) 10 (66.7%) |
| No of prior Tx (mean, range) | 2.4 (0-8) | 3.2 (0-7) | 2.7 (0-8) |
| FLIPI (n, %) 0-1 2 ≥ 3 | 1 (10%) 2 (20%) 7 (70%) | 1 (20%) 2 (40%) 2 (45%) | 2 (13.3%) 4 (26.7%) 9 (60%) |
| Positive GELF criteria (n, %) | 4 (40%) | 1 (20%) | 5 (30%) |
| . | Arm A (n = 10) . | Arm B (n = 5) . | Total (n = 15) . |
|---|---|---|---|
| Age (median, rage) | 64 (55 – 80) | 60 (31 – 86) | 63 (31 – 86) |
| Gender (M/F) | 6/4 | 2/3 | 8/7 |
| Histology (n, %) Follicular Marginal zone DLBCL | 10 (100%) 0 (0%) 0 (0%) | 3 (60%) 1 (20%) 1(20%) | 13 (86.6%) 1 (6.7%) 1 (6.7%) |
| Stage (N, %) II III IV | 1 (10%) 1 (10%) 8 (80%) | 2 (40%) 1 (20%) 2 (40%) | 3 (20%) 2 (13.3%) 10 (66.7%) |
| No of prior Tx (mean, range) | 2.4 (0-8) | 3.2 (0-7) | 2.7 (0-8) |
| FLIPI (n, %) 0-1 2 ≥ 3 | 1 (10%) 2 (20%) 7 (70%) | 1 (20%) 2 (40%) 2 (45%) | 2 (13.3%) 4 (26.7%) 9 (60%) |
| Positive GELF criteria (n, %) | 4 (40%) | 1 (20%) | 5 (30%) |
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.


This feature is available to Subscribers Only
Sign In or Create an Account Close Modal