Abstract
Background: The use of lenalidomide is expanding to include patients with refractory or relapsed diffuse large B cell lymphoma (DLBCL). Lenalidomide is less toxic than thalidomide, but it leads to hypothyroidism in approximately 5-10% of patients that are treated with this medication. The mechanism of lenalidomide-induced hypothyroidism is poorly understood, and it is important to better understand this phenomenon as its new applications in DLBCL portend much broader use.
Methods: In this study we compared rates of therapy-induced hypothyroidism in 329 consecutive patients diagnosed with DLBCL between 2000 and 2013 who were treated at Vanderbilt University Medical Center with conventional chemotherapy (DLBCL-c) or conventional chemotherapy plus lenalidomide (DLBCL-len). No patients were excluded for any reason, and our study protocol was approved by our institutional review board. We also measured serum levels of tumor necrosis factor alpha (TNF-α), interferon gamma (IFN-γ), interleukin-6 (IL-6), interleukin-12 (IL-12), and interleukin-15 (IL-15) in 27 patients before and after treatment with lenalidomide. The student’s t test and Mann-Whitney U rank sum test were used to compare continuous variables. For discrete variable comparisons the X2-test or Fisher’s exact test were used. All statistical analyses were performed with SPSS.21 software.
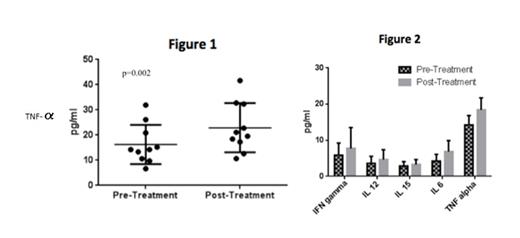
Results: Amongst our 298 patients with DLBCL who were not treated with lenalidomide (DLBCL-c group), the median age was 60 years (range 17-97 years) and 181 (61%) were male. Amongst our 31 patients treated with lenalidomide (DLBCL-len) the median age was 56 years (range 29-85 years) and 15 (48%) were male. A larger proportion of patients treated with lenalidomide had high-risk disease by international prognostic index (IPI) ≥3 (80.5% in the DLBCL-len group compared to 33.5% in the DLBCL-c group). In the DLBCL-c group only four patients (1.3%) developed hypothyroidism compared to eight patients (25.8%) in the DLBCL-len group (p<0.0001). The median time to onset of hypothyroidism in patients treated with lenalidomide was 5.2 months, and of the eight patients who developed new hypothyroidism five had National Cancer Institute (NCI) Common Terminology Criteria for Adverse Events (CTCAE) version 4.0 grade 2 toxicity and three had grade 3 toxicity. All patients who developed hypothyroidism achieved normalization of their thyroid function tests within a median of four months with levothyroxine treatment. The cytokine analysis revealed a statistically significant increase in serum TNF-α amongst the ten patients treated with lenalidomide who experienced new (eight patients) or worsening (two patients) hypothyroidism (mean TNF-α level 16.2 pg/mL pre-treatment and mean TNF-α level 22.9 pg/mL post-treatment, p=0.002, Figure 1 ). Amongst all 27 patients treated with lenalidomide for whom serum analysis was completed, including patients who did not develop hypothyroidism, there was no significant change in pre- and post-treatment serum TNF-α, IFN-γ, IL-6, IL-12, and IL-15 (Figure 2).
Conclusion:
More investigation is needed to describe the mechanism of lenalidomide-induced hypothyroidism in patients with DLBCL, especially as its treatment indications expand. We found higher rates of hypothyroidism when patients with DLBCL were treated with lenalidomide in addition to standard chemotherapy, and our cytokine analysis suggests that this is mediated by TNF-α. We recommend close monitoring of thyroid function tests in patients with DLBCL treated with lenalidomide.
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal