Abstract
Autologous stem cell transplantation (ASCT) is a frequently applied treatment modality for patients with multiple myeloma (MM) and (relapsing) malignant lymphomas. Normal peripheral blood cell counts are usually observed 1 year post ASCT although the hematopoietic stem cell (HSC) compartment is severely impaired reflected by reduced HSC frequency and quiescence (Haematologica 2013;98:1264). Since HSCs interact intensively with the surrounding microenvironment in the bone marrow and strongly depend on these cells for a proper function, we studied the mesenchymal stem cell (MSC) compartment in patients 1 year post ASCT.
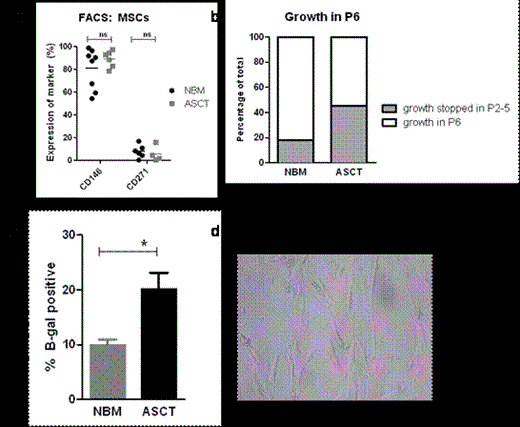
We generated a biobank with patient material acquired 1 year after ASCT. Immunohistological studies of bone marrow biopsies post ASCT showed increased expression of CD271 (Nerve Growth Factor Receptor, NGFR) compared to normal bone marrow (NBM, 11.26%±1.2 of bone marrow area versus 1.87%±0.9, p<0.0001) while no difference was observed for the percentage of nestin+ or perivascular CD146+ (Melanoma Cell Adhesion Molecule, MCAM) cells. In addition an increase in CD271+-multilocular adipocytes was noted, reflecting a difference in preferential MSC differentiation. Subsequently MSCs were cultured from the CD34- fraction of the bone marrow mononuclear cells, obtained from post ASCT patients (n=11) and compared to healthy subjects (n=17). MSCs were selected by their plastic-adherency and subsequently replated to generate MSCs. Cultured MSCs from post ASCT and NBM had similar population doubling times (1.92±0.22 and 3.52±1.02 in P4 (passage 4) respectively). In addition no difference in cell surface expression of CD146 and CD271 was demonstrated on MSCs post ASCT as compared to NBM. However, the post ASCT MSCs showed a change in morphology at early passages (P3-4) and premature exhaustion of growth in 45% of the studied patients (n=11) at P5, in contrast to 18% from NBM (n=11). B-galactosidase staining of post ASCT MSCs was increased in P5 and P6 compared to NBM MSCs (20.08%±3.0 vs 9.9%±1.1, p=0.04). To study the functionality of these MSCs, post ASCT MSCs from a low passage (P3 or P4) were used for co-culture experiments with CD34+ cord blood cells in the presence of cytokines SCF, FLT3 and TPO. Co-cultures with MSCs from different post ASCT patients showed a large variation in number of cobblestone-area forming cells (CAFCs, range: 11-163, mean: 81.3±16.0) as well as the size of cobblestone area. This reflects the diversity in HSC support by post ACST MSCs and concurs with the diversity found between patients in the clinical setting. Finally gene profiling performed on cultured post ASCT (n=10) and NBM (n=9) MSCs in early passages (P2 and P3) showed upregulation of proinflammatory genes such as interleukin-6 (IL6) and genes involved in Notch and Transforming Growth Factor-ß (TGF-B) signaling such as Hairy and Enhancer of Split-1 (HES1), and Bone Morphogenetic Protein (BMP)1 and BMP4. These findings were confirmed by quantitative PCR. Foxc1 expression, recently linked to maintenance of hematopoietic stem and progenitor cells, was significantly increased in post ASCT MSCs.
Collectively, these data indicate changes in the bone marrow niche, especially in the mesenchymal (CD271+) compartment, inducing premature exhaustion and affecting their supportive role for the HSCs. This damage to the niche may account for the reduced bone marrow reserve observed in patients and generate insight into putative therapeutic targets for improving transplantation strategies.
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.


This feature is available to Subscribers Only
Sign In or Create an Account Close Modal