Abstract
Coversin is a clinical stage recombinant protein molecule (16.7kDa) derived from a salivary molecule from the Ornithodros moubata tick where it assists the parasite to feed without provoking a host immunological response. It prevents activation of complement C5 and binds leukotriene B4 (LTB4) with high affinity. It is not an antibody and can therefore be manufactured in prokaryotic cells such as E. coli leading to potential savings in manufacturing costs. As its mode of action on C5 is similar to that of the monoclonal antibody eculizumab (Soliris®), it is considered to be a promising alternative therapeutic approach to thrombotic and hemolytic diseases mediated by complement activation including atypical hemolytic uremic syndrome (aHUS), catastrophic anti-phospholipid syndrome (APS) and paroxysmal nocturnal hemoglobinuria (PNH).
In order to test this hypothesis, experiments were undertaken to determine whether Coversin could inhibit the hemolysis of PNH red cells in vitro using a modified Ham test combined with visualisation of the results by flow cytometric measurement of CD59 expression. Initial experiments showed maximum inhibition of hemolysis compared to control occurred at a concentration of approximately 10mcg/mL. Further experiments using PNH red blood cells also demonstrated the effectiveness of Coversin in blocking in vitro hemolysis of PNH red cells that had both type III (complete GPI-deficiency) and type II (partial GPI deficiency) red cells and also the deposition and accumulation of C3d on both types of PNH red cells. In a comparative experiment Coversin 10mcg/mL was found to be as effective as eculizumab 50mcg/mL, a molar equivalent dose.
As Coversin is a non-humanised xenologous protein a mouse study was performed in order to assess immunogenicity and its potential to induce the formation of neutralising antibodies. Daily repeat subcutaneous doses of Coversin (0.57mg/kg) or vehicle were administered to 5 groups of 6 BALB/c mice and blood samples taken after 7, 14, and 28 days administration with an additional group where samples were taken 14 days following cessation of 28 days dosing. Immunoblots showed that by Day 14 33% of mice had developed low titre IgG antibodies to Coversin. By 28 days this had risen to 75% of mice receiving the active compound. Using CH50 lytic assays the antibodies were shown to be non-neutralising and no animals exhibited clinical signs of allergy or injection site reactions. The group of 6 mice that received vehicle showed no evidence of antibodies at 28 days.
A heterozygous C5 mutation leading to a p.Arg885His polymorphism and interfering with the binding of eculizumab was recently reported in Japanese PNH patients and this was associated with a poor response to therapy. This was subsequently found to affect 3.5% of the general Japanese and Han Chinese population. A similar polymorphism has now been shown in a 4 year old Caucasian child with persistent thrombocytopenia following a stem cell transplant for chronic granulomatous disease. Prolonged eculizumab treatment was ineffective leading to discovery of the mutation. Serum from the patient was spiked with ascending concentrations of Coversin and CH50 activity was measured by ELISA. This produced a dose response curve corresponding to that found in serum from normal subjects. Coversin, which binds to a slightly different site on the C5 molecule, seems therefore to be unaffected by the polymorphism and to achieve superior inhibition of C5 activation compared to eculizumab.
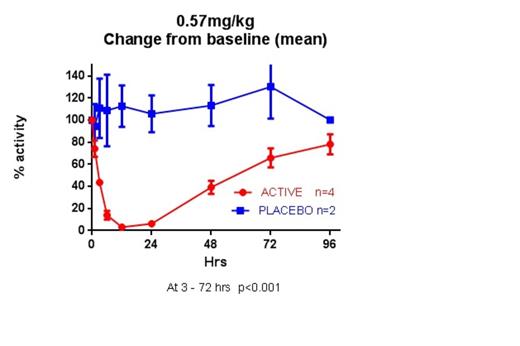
A Phase I, single ascending dose clinical trial was performed in 24 healthy volunteers. In the top dose cohort total blockade of complement C5 was achieved following subcutaneous injection at the predicted therapeutic loading dose of 0.57mg/kg. Activity remained below 50% for 48 hours suggesting that dosing once a day is feasible at steady state (see figure). The drug was well tolerated and no drug-related side effects were seen. Phase II clinical trials in PNH and other thrombotic micro-angiopathies (TMAs) are planned.
Coversin appears to be a promising alternative to eculizumab for patients with PNH and other TMAs including those with polymorphisms which interfere with optimal binding of eculizumab to complement C5.
Weston-Davies:Volution Immuno Pharmaceuticals (VIP) SA: Consultancy. Nunn:Volution Immuno Pharm: Consultancy. Prudo:Volution Immuno Pharmaceuticals (VIP) SA: Equity Ownership, Membership on an entity's Board of Directors or advisory committees.
Author notes
Asterisk with author names denotes non-ASH members.
This icon denotes a clinically relevant abstract

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal