Abstract
Introduction: Many cardiovascular procedures including percutaneous coronary intervention, coronary bypass surgery, and peripheral vascular surgery require antithrombotic therapy during the procedure. Heparin is frequently used, due to low cost, ease of monitoring and reversibility with protamine. Antiplatelet agents such as aspirin and clopidogrel are also frequently used but these agents are not reversible. Alternative reversible antithrombotic strategies that interfere with platelet-mediated thrombosis are needed. We are investigating the use of a reversible agent that is specific for von Willebrand factor (VWF) for use during cardiovascular procedures.
We previously developed an antidote-controlled 80 oligonucleotide RNA aptamer Ch-9.14-T10 (T10). (Nimjee, S.M., Lohrmann, Wang, H, et al. Molecular Therapy 2012; 2:391-397). T10 bound to VWF with high affinity and inhibited thrombosis in a murine carotid artery damage model. The aptamer also greatly increased bleeding in mice that were surgically challenged by tail transection and blood loss decreased when complementary antidote oligonucleotides were administered. In addition, T10 inhibited primary hemostasis in the PFA-100 assay and this inhibition was completely reversed by the antidote. This study further characterizes the T10 aptamer, as well as a truncated version of the T10 aptamer, using clinical laboratory assays that would be used to monitor antithrombotic therapies during cardiovascular procedures.
Methods: We initiated our study with T10 and then developed a truncated version, T25, a 60 oligonucleotide with which we performed additional investigations. We recruited normal donors for analysis of aptamer and antidotes. All participants had normal platelet counts, VWF activity and antigen levels, and were not taking antiplatelet agents. Laboratory tests included: ristocetin induced platelet agglutination (RIPA), VWF activity, ristocetin cofactor activity, cone and plate(let) analyzer, thromboelastography (TEG) and thromboelastometry (ROTEM).
Results: We first determined the effects of the VWF aptamers on RIPA, VWF activity, and ristocetin cofactor activity to confirm functional inhibition of VWF. We found that T10 (500-750 nM) and T25 (250-750nM) completely inhibited RIPA depending on the donor. T25 also inhibited VWF activity with a Ki of 160-320 nM and ristocetin cofactor activity with an IC50 of 3000 nM. Employing a cone and plate(let) analyzer we found that T25 (750 nm) reduced platelet adhesion by 33-49% on a polystyrene surface under arterial flow conditions (1800 s-1, for 2 minutes). The aggregate size was also reduced by 30%.
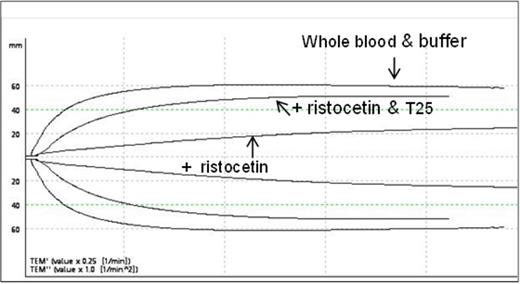
As ACT and TEG are frequently used during cardiovascular procedures, we performed several measures with our VWF aptamer. T10 had no effect on the ACT (730-2000nM), TEG (1600 nM), or ROTEM (750 nM). Standard methods were used for the TEG (Kaolin activation followed by CaCl2) and ROTEM (EXTEM assay) Topf et al. reported a modified ROTEM protocol where they found that incubating citrated whole blood with ristocetin decreased the clot strength as measured by the maximum amplitude (Thromb.Haemost 2011; 105:1091). We reasoned that an aptamer that bound to VWF would inhibit the effect of ristocetin on the maximum amplitude. We found that T25 did inhibit the effect of ristocetin and titration studies showed that 200-400 nM T25 was needed to restore the maximum amplitude to at least 80% of its original value. An example of the ROTEM tracings is shown in figure 1.
Conclusion: The VWF-specific aptamer T25 can be detected by several clinical assays, including the RIPA, VWF activity assays, and the cone and plate(let) analyzer. It is not detectable by an ACT or standard TEG or ROTEM, however. We are able to detect the aptamer using a modified ROTEM and are further defining the adaptability of this assay to facilitate monitoring during cardiovascular procedures.
Citrated whole blood from was incubated with buffer or ristocetin (0.7 mg/ml) or ristocetin and T25 (300 nM) as indicated. Clotting was initiated with EXTEM (tissue factor pathway activation) and CaCl2.
VWF Aptamer T25 Restores the Decrease in Clot Strength by Ristocetin in Citrated Whole Blood from a Normal Donor.
VWF Aptamer T25 Restores the Decrease in Clot Strength by Ristocetin in Citrated Whole Blood from a Normal Donor.
Becker:Regado Biosciences: Membership on an entity's Board of Directors or advisory committees.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal