Abstract
Hematopoietic stem cell transplantation (HSCT) is the best treatment option for young patients with aplastic anemia. Conditioning with fludarabine (Flu) and cyclophosphamide (Cy) has been associated with improved long-term survival in patients undergoing HSCT (George et al, 2013). Graft versus host disease (GvHD), graft failure and infection associated mortality are still some of the major hurdles towards a successful outcome. Limited data available on the association between plasma Flu levels and HSCT outcome showed higher plasma Flu AUC levels to be a risk factor for non-relapse mortality. Fludarabine given intravenously as fludarabine mono-phosphate is readily converted to Flu by the enzyme ecto-5'-nucleotidase or NT5E and then is taken up into the cells by nucleoside transporters. NT5E mRNA expression and gene polymorphism in the lymphoblastoid cell lines have been shown associated with cytotoxicity to thiopurine drugs but its role on Flu pharmacokinetics (PK) is not known. We proposed to evaluate the population PK of Flu in patients with aplastic anemia undergoing HSCT and to test their influence on transplant outcome.
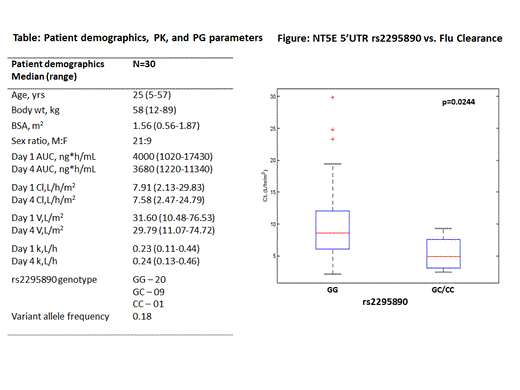
Thirty patients diagnosed with aplastic anemia and consented to undergo HLA identical sibling HSCT using a Flu/Cy based conditioning regimen between January 2012 and February 2014 at the Department of Hematology, Christian Medical College, Vellore were included in the study (Table: Patient demographics). Pre-HSCT DNA was used to screen polymorphisms in the NT5E gene. Plasma was separated from the peripheral blood collected before (0hr) and 1,2,3,5,7 and 24 hrs after the infusion of fludarabine (30mg/m2/day x 6 days over 1 hr infusion) on days 1 and 4 and stored at -80oC until further analysis. Plasma fludarabine was analyzed using a LC-MS/MS based method and the concentration was expressed as ng/ml. Flu PK was estimated using a 2-compartment model with linear elimination. Inter-occasion variability (between dose 1, 4, 5, and 6 PK studies) on the volume (V) and elimination rate (k) was accounted for in the model. The covariates tested were: age, sex, body weight, BSA, disease type, and a polymorphism in NT5E gene. The population PK was analyzed using Monolix 4.3.2. The PK parameters AUC, CL, V and k were calculated for day 1 and day 4 of sampling. A limited sampling model (LSM) was developed with this data in order to reduce sample collection time points for future studies. The PK and PG parameters estimated are listed in Table. The influence of flu PK on clinical outcome parameters including overall survival, GvHD and rejection were estimated using Cox regression analysis.
Flu PK showed wide inter-individual variation in patients with aplastic anemia receiving fludarabine based conditioning regimen (Table), which could be explained by the polymorphism in the gene converting the prodrug to active form of fludarabine. The NT5E 5’UTR variant rs2295890 was in complete linkage disequilibrium with 4 other SNPs namely: rs9450278, rs9450279, rs4599602, rs4458647 as seen both in patients and in normal healthy volunteers. Patients with variant genotype for rs2295890 showed significantly lower plasma Flu clearance compared to those with wild type genotype (p=0.0244) (Figure). Comparison of Flu PK parameters with previous studies is not possible due to heterogeneous population of patients, Flu dose and donor type included (Long Boyle et al, 2011; McCune et al, 2012). The LSM developed based on the current PK and using a D-optimality approach (via ADAPT 5) suggested a 4 time point sampling including 1 hr (end of infusion), 3, 7, and 24 hrs as a reasonable approach. This LSM will be validated by analyzing future samples.
Of the 30 patients, 26 (86.6%) engrafted at a median of 14 days post BMT (range: 11-18) while 2 (6.6%) had primary graft failure and 2 died within the first 2 weeks of transplant. Grade 2-4 acute GVHD was seen in 6 (25%) with chronic GVHD in 28.5%. One patient had secondary graft failure. The Day 100 mortality was 26.6% and at present 19 (63.3%) are alive. None of the Flu PK parameters showed any significant association with engraftment, GVHD, rejection and mortality.
This first study on Flu PK in a uniform cohort of patients with aplastic anemia undergoing HSCT shows that the PK of Flu is highly variable and there is genetic basis for this variation. The clinical significance of the variation in the Flu PK needs to be studied in a larger cohort of patients.
Srivastava:Octapharma: Consultancy, Other.
Author notes
Asterisk with author names denotes non-ASH members.
This icon denotes a clinically relevant abstract

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal