Abstract
Background: Infants <1 year of age with AML are at high risk of early death during induction, pulmonary and infectious toxicities, and treatment related mortality (TRM). Compared with older children, infants are more likely to have higher risk clinical and cytogenetic features. Targeted therapy is needed to improve the outcomes for infants with AML while minimizing toxicities.
Objective: To determine if the addition of gemtuzumab ozogamicin (GO) to standard chemotherapy is safe, tolerable, and improves event free survival (EFS) in infants <1 year of age with AML.
Methods: Infants >/= 1 month to <1 year of age with de novo AML or <1 month of age with progressive AML were eligible for enrollment on the COG trials AAML03P1 and AAML0531. Infants with acute promyelocytic leukemia, juvenile myelomonocytic leukemia, bone marrow failure syndromes, or Down syndrome were not eligible. The 5-course chemotherapy backbone was identical in both trials. GO 3 mg/m2/dose (0.1 mg/kg for patients with a body surface area <0.6 m2) was given on day 6 of Induction (Ind) I and day 7 of Intensification (Int) II to all patients on AAML03P1 and to randomized patients on the experimental arm of AAML0531. Patients on the control arm of AAML0531 were treated with standard therapy without GO (noGO). Stem cell transplant (SCT) was given following IntI to patients on AAML03P1 with a matched family donor (MFD) and to patients on AAML0531 with high risk (HR) disease or with intermediate risk (IR) disease and a MFD. SCT recipients only received one dose of GO. Patients were removed from protocol if the bone marrow had >/= 20% blasts after IndI on AAML03P1 or >/= 5% blasts after IndII on both studies. Early death (ED) was defined as death during IndI.
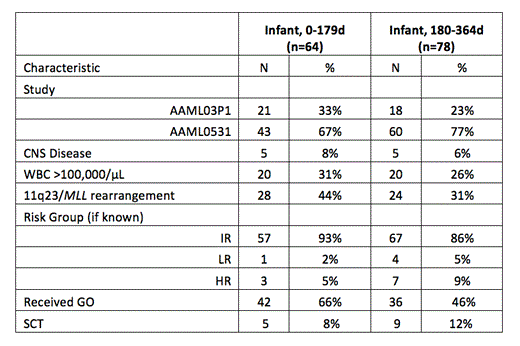
Results: AAML03P1 enrolled 39 infants from 2003-2005 and AAML0531 enrolled 103 infants from 2006-2010 (Table 1). Median follow up was 5.02 years. The demographics and disease characteristics of infants enrolled on both studies were similar. Compared with enrolled children >/= 1 year of age, infants had higher frequencies of hepatomegaly (p<0.001), splenomegaly (p<0.001), hyperleukocytosis (WBC>100x103/µL, p=0.003) and French-American-British (FAB) M5 (p<0.001), and M7 (p<0.001) AML. Infants had less FAB M1 (p<0.001), M2 (p<0.001), and M4 (p=0.029) AML. There was no difference in CNS disease by age. The frequency of 11q23/MLL rearrangement was highest in infants 0-179 days of age (44%) and significantly decreased with increasing age (p<0.001). Favorable cytogenetics were rare in infants: t(8;21) was not found and inv(16)/t(16;16) was only found in 4 patients. FLT3-ITD HAR was absent in infants. The majority of infants (89%) fell into the IR group and infants were less likely to have had high (HR) or low risk (LR) disease when compared with children >/= 1 year.
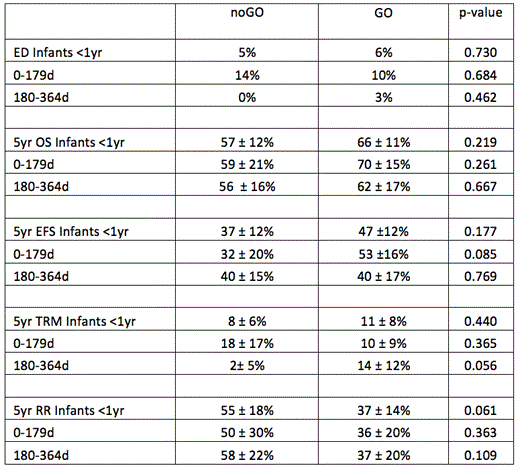
The ED rate was higher in younger infants (7 ED, infants 0-179d) when compared with older infants (1 ED, infants 180-364d) (p=0.013). EDs were not increased in infants who received GO vs. noGO (5 vs. 3 ED, p=0.730). The complete remission (CR) rate for infants was 68% at the end of IndII. The 5 year EFS was lower in infants than children >/= 1 year (42 vs. 50%, p=0.001). The 5 year OS for infants was 62%, relapse risk (RR) was 44%, and TRM was 10%. Infants who received GO had improved OS, EFS, and RR, though the differences were not statistically significant (Table 2).
Combined outcomes of infants on AAML03P1 and AAML0531: noGO vs. GO Conclusion: GO in combination with intensive chemotherapy is tolerable in infants with AML, does not increase early deaths, and is associated with trends toward improved EFS, OS, and RR.
Combined outcomes of infants on AAML03P1 and AAML0531: noGO vs. GO Conclusion: GO in combination with intensive chemotherapy is tolerable in infants with AML, does not increase early deaths, and is associated with trends toward improved EFS, OS, and RR.
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal