Abstract
Aim:
The first analysis of the ALFA-0701 study, testing the addition of gemtuzumab ozogamicin (GO) to standard treatment in de novo AML patients aged 50 to 70 years, was presented at ASH 2011 and showed a benefit in 2-year estimated EFS (primary endpoint of the study) (P=0.0018), RFS (P=0.0009) and OS (p=0.03). With a longer median follow up for alive patients of 43 months (reference date: 04/30/2013), we present here the final analysis of this study.
Methods:
Patients were randomized to receive induction therapy with daunorubicin (DNR) 60 mg/m2 for 3 days and cytarabine 200 mg/m2 for 7 days ± fractionated doses of GO (3mg/m2 on days 1, 4, 7). Patients in CR/CRi received 2 consolidations courses of intermediate doses of cytarabine ± one dose of GO (3mg/m2on day 1, maximum dose: 5mg), according to initial randomization. From March 2008 to November 2010, 278 patients were included.
Results:
CR+CRi was observed in 215/278 patients, 103/139 in control and 112/139 in GO arm (p=0.25). Primary resistant patients were 30/139 in control and 18/139 in GO arm (p=0.08). There were 6/139 (4%) induction deaths in control and 9/139 (6%) in GO arm (P=0.41).
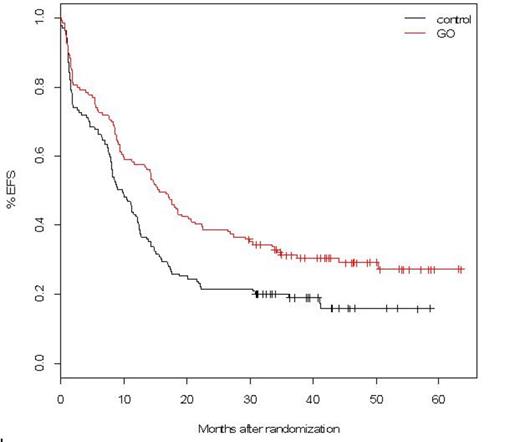
In this final analysis, with follow-up ranging from 30 up to 63.5 months in alive patients, the EFS and RFS benefits associated with GO were still observed, with estimated 3-year EFS at 19% in control versus 31% in GO arm (HR=0.66, 95%CI: 0.50-0.87; median: 9.7 vs. 15.6 months; p=0.0026) and 3-year RFS at 25% versus 38% (p=0.006). However, OS data did not confirm benefit with 3-year OS at 36% in control and 44% in GO arm (HR=0.82, 95%CI: 0.60-1.10; median: 20.8 vs 25.4 months; p=0.18). At the reference date, 132 patients had relapsed (69 control, 63 GO). After relapse, most patients received either intensive salvage with idarubicin and cytarabine (28 control, 42 GO) or GO as a single agent or in combination (20 control, 1 GO). A minority of patients received azacytidine (10 control, 8 GO) or supportive care (11 control, 12 GO). Second CR rate was 66% after intensive salvage, 47% after GO-containing salvage, and 5% after azacytidine. Cumulative incidence of second CR (42% in control and 51% in GO arm) did not differ according to the randomization group (p=0.38). Overall survival after relapse did not differ according to the randomization group. There was no evidence of difference in survival after relapse among those patients who received intensive or GO-containing salvage (2-year post-relapse OS, 31% and 37% respectively).
Overall throughout the study, 47 patients in control and 33 in GO arm received an allogeneic stem cell transplant (SCT) either as primary resistant patients (6 and 3, respectively) or in CR1 (23 and 17, respectively) or after relapse (18 and 13, respectively). The cumulative incidence of SCT did not differ significantly between the two arms (p=0.29). Post-SCT survival did not differ between the two arms.
Prognostic analyses were performed including age, gender, baseline WBC, ECOG, cytogenetics and the following gene mutations: NPM1, FLT3-ITD, IDH1, IDH2, RUNX1 and DNMT3A. Univariate prognostic analyses for EFS showed that besides treatment arm, cytogenetic and DNMT3Amutation were the only prognostic factors identified. After adjustment on these factors, GO arm remained significantly associated with longer EFS (p=0.013).
The effect of treatment arm was then analyzed in the patient subsets: age (60-year cutoff), cytogenetics, FLT3-ITD, NPM1 and DNMT3Amutations. No significant heterogeneities were observed across these subsets for EFS or OS and the tests for interaction were negative, though the effect of GO was not observed in patients with unfavorable cytogenetics.
In terms of safety, 68 and 101 SAEs were reported in 60 (43%) and 79 (57%) patients, in control and GO arm respectively. More patients from GO arm experienced more than one SAE (P= 0.031). Persistent thrombocytopenia (<50.10.9/L) by day 45 after induction or consolidation were reported in 21 patients (15%) in the GO arm compared to 4 patients (2%) in the control arm (P= 0.0001).
Conclusion. Final analysis of the ALFA 0701 study with a longer follow up confirms the efficacy of adding GO to standard chemotherapy in AML treatment on primary endpoint EFS and on RFS. However, the gain in OS was no longer observed. After relapse, a similar number of patients reached second CR and were transplanted in second CR in the two arms. Of note, one third of patients from the control arm received GO at the time of relapse.
Castaigne:Pfizer Inc: Consultancy, Honoraria. Off Label Use: Gemtuzumab Ozogamicin is not authorized for previously untreated AML..
Author notes
Asterisk with author names denotes non-ASH members.
This icon denotes a clinically relevant abstract

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal