Abstract
Immunotherapy targeting individual antigens in acute myeloid leukemia (AML) has shown promise. However, in view of leukemia heterogeneity and the loss of tumor antigen expression by AML, it is unlikely that any single antigen will be consistently expressed by all leukemia cells. This highlights the need to identify additional antigens in AML that can be targeted.
Azurophil granule proteases have been shown to be effective immunotherapeutic targets. Proteinase 3 and neutrophil elastase, the parent proteins for the HLA-A2 restricted peptide PR1, have been targeted successfully in myeloid leukemia using immunotherapy. We recently discovered the HLA-A2 restricted peptide CG1 (FLLPTGAEA), which is derived from the azurophil granule protease cathepsin G (CG). We showed that CG is highly expressed by AML blasts and leukemia stem cells in a limited number of AML samples and showed that CG1 can be targeted in vitro using CG-specific cytotoxic T lymphocytes (CTL).
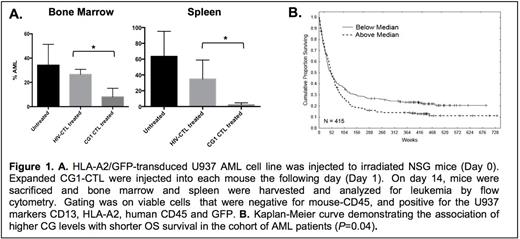
We sought to determine whether CG1 can be targeted in vivo and to characterize the expression of CG in a large cohort of AML patients. To assess the efficacy of targeting CG1 in vivo, we used a NOD scid gamma (NSG) mouse model engrafted with the human HLA-A2+ AML cell line U937 (U937-A2), which is known to express CG. Mice were injected with U937-A2 (0.5 x 106) cells and on the following day were treated with either CG1-CTL (0.25 x 106), negative control HIV-CTL expanded from the same donor or were left untreated. Mice treated with CG1-CTL demonstrated a significantly greater reduction in U937-A2 in the bone marrow (BM) (8% residual AML; P<0.05; Figure 1A) after 2 weeks compared with mice treated with HIV-CTL (27% residual AML) or that were left untreated (34% residual AML). A similar reduction in AML was seen in the spleens of mice treated with CG1-CTL. Since normal myeloid cells also express CG, to demonstrate the specificity of CG1-CTL for AML, we engrafted NSGS mice with human HLA-A2+ cord blood (CB). After confirming engraftment by detecting human (h) CD45+/HLA-A2+/mouse (m)CD45- cells in peripheral blood, we treated mice with CG1-CTL or HIV-CTL (0.25 x 106). A similar percentage of hCD45+/HLA-A2+/mCD45- cells was detected in the BM of CB-engrafted mice in both CG1-CTL (24% CB) and HIV-CTL (27% CB) after 6 weeks, suggesting the safety of targeting CG1.
After demonstrating the efficacy of targeting CG1 in vivo, we then studied CG1 presentation and CG expression in AML patients. To determine CG1 presentation, primary AML blasts, U937-A2 and HLA-A2-trasnduced HL60 (HL60-A2) cell lines were lysed then immunoprecipitated with anti-HLA-A2. Peptides were eluted using standard acid elution methodology and then analyzed by mass spectrometry. CG1 peptide was detected in 6 of 9 primary AML samples and in HL60-A2 and U937-A2 cells. Reverse phase protein analysis (RPPA) was used to study CG protein expression in a large cohort of AML patients and normal donors (ND) to include the following: 511 newly diagnosed patients, 21 ND peripheral blood leukocyte controls, 21 ND CD34+ cell controls and 10 ND BM controls. CG expression in the 511 AML patients samples demonstrated a Gaussian distribution and was higher than CG expression by ND CD34+ cells in 230 samples, equal to normal CD34+ cells in 234 samples and less than normal CD34+ cells in 47 samples. As expected, G-CSF primed ND CD34+ cells had high expression of CG. There were no significant differences in CG expression within the different AML FAB subtypes, although M4 eos and M5b subtypes showed the lowest expression levels. In multivariate Cox model analysis, higher CG expression significantly predicted shorter overall survival (OS) in all patients (P=0.04) (Figure 1B). The association of CG with shorter OS was most significant in patients who had intermediate cytogenetics and FLT3 mutation (P=0.02). Moreover, CG levels were determined in paired samples taken at diagnosis and relapse, and the levels were compared using paired t test and Pearson product-moment correlation analysis. Among 47 cases with paired diagnosis and relapse samples, there was significantly higher CG protein expression in relapse samples (P=0.0001).
In summary, we found CG to be an important antigen in AML. CG is associated with worse outcomes in AML patients, however in vivo mouse data show that targeting CG eliminates AML while sparing normal hematopoiesis. Together these data indicate that CG has a promising role as a novel immunotherapeutic target in AML.
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal