Abstract
Background: Oprozomib (OPZ) is a selective oral proteasome inhibitor that binds selectively and irreversibly to its target. In a phase 1b/2 study of single-agent OPZ in patients (pts) with hematologic malignancies, OPZ has shown promising antitumor activity in pts with multiple myeloma (MM) (Savona, ASH 2012, 203; Kaufman, EHA 2013, P223; Ghobrial, ASH 2013, 3184). This multicenter, single arm phase 1b/2 study evaluates the safety and tolerability of OPZ with dexamethasone (DEX) in pts with relapsed and/or refractory MM. Initial results from the study are presented.
Methods: This is an open-label, phase 1b/2 study (NCT01832727). Pts with relapsed and/or refractory MM who have received 1–5 prior lines of therapy (at least 1 regimen including lenalidomide and/or bortezomib) are eligible for enrollment. Pts are receiving OPZ on days 1, 2, 8, and 9 of a 14-day cycle (2/7 schedule) or on days 1–5 of a 14-day cycle (5/14 schedule). The starting OPZ dose was 210 mg on both schedules. Doses are being escalated in 30-mg increments using a standard 3+3 dose-escalation scheme. DEX (20 mg) is being given by oral administration on days 1, 2, 8, and 9 of a 14-day cycle. Treatment is being administered until pt withdrawal or progressive disease. The primary objectives of the phase 1b study are to determine the maximum tolerated dose (MTD) and the recommended phase 2 dose (RP2D) of OPZ with DEX and to evaluate safety and tolerability. Response is being assessed by IMWG criteria, with inclusion of minimal response and near complete response by modified EBMT criteria to provide an overall response rate (ORR) as reflected by partial response (PR) or better and a clinical benefit rate (CBR) as reflected by minimal response (MR) or better.
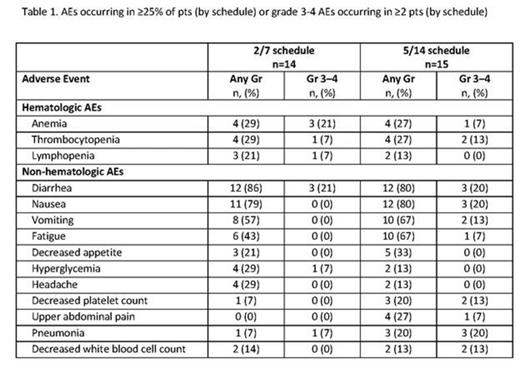
Results: As of July 7, 2014, 29 pts were enrolled (2/7 schedule, n=14; 5/14 schedule, n=15). Median age was 64 years (2/7 schedule) and 63 years (5/14 schedule). Pts received a median of 3 prior regimens in the 2/7 schedule and 2 prior regimens in the 5/14 schedule. Preliminary median OPZ treatment duration in this ongoing study was 13.3 weeks in the 2/7 schedule (range, 3.3–35.3 weeks) and 5.7 weeks in the 5/14 schedule (range, 0.1–24.7 weeks). No dose-limiting toxicities (DLTs) have occurred in pts on the 2/7 schedule; 3 DLTs were observed on the 5/14 schedule (210 mg cohort; grade 2 subarachnoid hemorrhage, grade 3 transaminitis, and grade 4 thrombocytopenia [n=1 each]). In both treatment schedules combined, the most common adverse events (AEs) were diarrhea (83%), nausea (79%), and vomiting (62%); additional AEs are shown in the table. The most common grade 3 AEs included diarrhea (21%), anemia (14%), pneumonia (14%), and nausea (10%). Grade 4 AEs included thrombocytopenia (n=1, 2/7 schedule; n=2, 5/14 schedule) and decreased lymphocyte count (n=1, 5/14 schedule). Grade 5 sepsis occurred in 1 pt on the 2/7 schedule (240 mg/d) and 1 pt on the 5/14 schedule (210 mg/d). Treatment was discontinued because of AEs in 2 pts on the 2/7 schedule and 7 pts on the 5/14 schedule. Five pts on the 2/7 schedule and 7 pts on the 5/14 schedule had their OPZ dose reduced at least once. In 12 pts enrolled on the 2/7 schedule who had ≥2 assessments for response, 5 pts had a PR, 2 pts had a MR, and 5 pts had stable disease (SD) as their best overall response for an ORR of 41.7% and a CBR of 58.3%. In 7 pts enrolled on the 5/14 schedule who had ≥2 response assessments, 3 pts had a MR and 2 pts had SD as their best overall response for a CBR of 42.9%.
Conclusions: Preliminary results suggest that treatment with OPZ and DEX has improved gastrointestinal tolerability in pts with relapsed and/or refractory MM relative to single-agent OPZ (Savona, ASH 2012, 203; Kaufman, EHA 2013, P223; Ghobrial, ASH 2013, 3184). Additional measures will be taken to improve gastrointestinal tolerability. Encouraging responses have been seen in this heavily pretreated patient population, especially with the 2/7 schedule. Enrollment in the phase 1 portion of the study is ongoing; dose escalation will continue until the MTD and RP2D are determined. Updated results will be presented at the meeting.
Hari:Genzyme: Consultancy, Research Funding; Millenium: Consultancy, Research Funding; Celgene: Consultancy, Research Funding; Onyx: Consultancy, Research Funding. Shain:Onyx/Amgen: Speakers Bureau. Voorhees:Millennium/Novartis: Membership on an entity's Board of Directors or advisory committees; Oncopeptides: Research Funding; Prolexys Pharmaceuticals: Research Funding; Acetylon: Research Funding; Janssen: Research Funding; GSK: Consultancy, Research Funding; Celgene: Consultancy, Research Funding. Abidi:Onyx: Speakers Bureau. Zonder:Celgene: Honoraria, Research Funding; Bristol-Myers Squibb: Honoraria. Richardson:Onyx: Membership on an entity's Board of Directors or advisory committees. Neuman:Onyx Pharmaceuticals: Employment. Dixon:Onyz Pharmaceuticals: Employment, Equity Ownership.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal