Abstract
INTRODUCTION
Previously, our work highlighted the synergistic activity of combining the selective inhibitor of nuclear export (SINE) selinexor with the irreversible proteasome inhibitor (PI) CFZ in multiple myeloma (MM) patient-derived plasma cells and cell lines, and in a murine xenograft model (Rosebeck et al, Blood 2013:122(21):279). Our studies were the first to suggest a role for both autophagy and apoptosis in response to combined SINE/PI treatment. Importantly, our results served as pre-clinical rationale for a currently-enrolling phase I clinical trial for the treatment of refractory and/or relapsed MM (NCT02199665). To gain insight into the molecular mechanism of synergy, we explored the novel role of caspase 10, which was implicated in our previous work, in the cytotoxic effects of combined KPT-330/CFZ treatment.
METHODS
MM cell lines were grown in RPMI1640/10% FBS. Subcellular fractionation was performed by three rounds of freeze-thaw in CHAPS lysis buffer. Caspase activity was assayed according to the manufacturer's instructions (BioVision). Otherwise, our studies used standard cellular and molecular biology techniques.
RESULTS
First, we demonstrate an increase in activity of caspases 10, 8, 9, and 3, but not caspase 1, in response to KPT-330/CFZ treatment. Inhibition of caspase 10 activity prevented activation of caspases 8, 9 and 3, abrogated cleavage of apoptotic substrates, including PARP and NF-κB, and significantly protected MM cells from the cytotoxic effects of SINE/PI treatment.
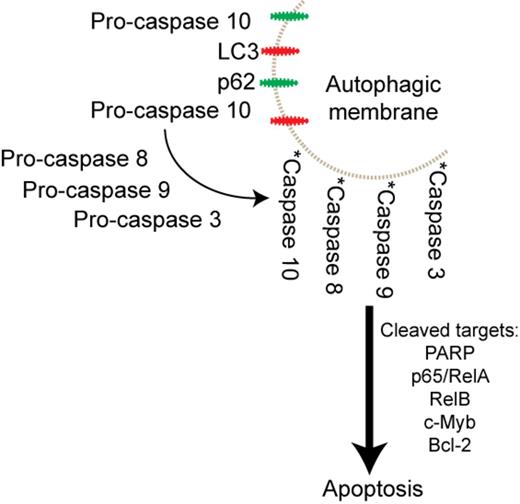
Caspases 8 and 10 are orthologs; caspase 10 is not present in mice and is not well-characterized. In response to PI, caspase 8 is recruited to autophagic membranes via interaction with Sequestosome1 (p62) and LC3, key autophagy components whose membrane localization is enriched upon PI treatment, creating an intracellular death-inducing signaling complex. Therefore, we hypothesized that, given the homology and functional overlap between caspases 8 and 10, the same could be possible of caspase 10. To test this, we performed subcellular fractionation to separate soluble cytoplasmic proteins from detergent-insoluble, membrane-embedded proteins and interrogated the relationship between caspase 10 and p62. In untreated cells we were struck by the presence of active fragments of caspases 10, 8, and 9, but not 3, in the insoluble fractions; full-length pro-caspases were found mostly in the soluble extracts. Selinexor/CFZ enhanced activation of all caspases and nearly the entire population of active caspases was found in the detergent-insoluble fractions. Total p62 levels were prominently induced by SINE/PI treatment and two cleavage fragments that correspond to the size of previously-identified in vitro caspase-cleaved fragments were found exclusively in the insoluble fraction. These data suggested that relocalization of caspase fragments to an intracellular membrane harnesses caspase activity.
Next, we used indirect immunofluorescence microscopy to assess the localization of caspases 10 and 8 and p62. Untreated cells showed diffuse staining, whereas selinexor/CFZ-treated cells had punctate staining and co-localization of caspases 10 and 8 with p62. Finally, in untreated cell lysates, GST-p62 pulled down endogenous LC3 and pro-caspase 10, but not its active fragments. Pro-caspase 10/GST-p62 association was dramatically reduced when using lysates from SINE/PI-treated cells, suggesting a priming effect of their association.
CONCLUSIONS
Our results are the first to demonstrate an intracellular mechanism of caspase 10 activation and suggest a model of synergy wherein selinexor/CFZ increase autophagic membranes embedded with p62 and LC3 that associate with pro-caspase 10 promoting its activation, likely by induced proximity, cleavage of other caspases and targets, and subsequent apoptosis (Figure). The nature of the novel intracellular membrane-embedded aggregate of active caspases requires further investigation.
McCauley:Karyopharm Therapeutics Inc.: Employment, Equity Ownership, Patents & Royalties. Shacham:Karyopharm Therapeutics: Employment. Kauffman:Karyopharm Therapeutics: Employment. Jakubowiak:Bristol Myers-Squib: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees; Celgene: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Janssen: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Novartis: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees; Millennium: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees; Onyx: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; SkylineDx: Honoraria, Membership on an entity's Board of Directors or advisory committees.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal