Abstract
A pivotal step during B-cell development is the expression of the precursor B-cell receptor (pre-BCR) by pre-B lymphocytes (cyto-Igµ+, surface-IgM-). The pre-BCR represents an immature form of the BCR and consists of two immunoglobulin heavy chains (IgH), two surrogate light chains (SLC) and the signal transducing adapter proteins Igα and Igβ. A functional pre-BCR drives proliferation of pre-B-cells, ensuring their further differentiation into mature B-cells. By immunophenotype, ~20% of B-cell acute lymphoblastic leukemia (B-ALL) cases originate from the pre-B-cell stage (pre-B-ALL) of lymphocyte development and might therefore also express the pre-BCR. In view of the importance of pre-BCR signaling for normal pre-B-cell development, we hypothesize that it is exploited by pre-B-ALL for malignant growth and proliferation.
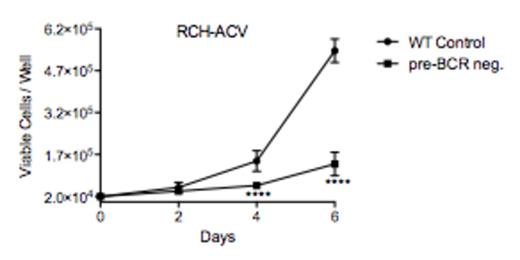
A hallmark of active pre-BCR signaling is the continuous internalization of pre-BCRs, resulting in low pre-BCR surface expression. Using this phenotype of active pre-BCR signaling (low pre-BCR expression and high phosphorylation of the pre-BCR associated kinases LYN and SYK), we identified pre-BCR+ ALL cell lines (RCH-ACV, SMS-SB and Nalm6) and xenograft expanded patient samples. To study the role of the pre-BCR in these cells, we rendered RCH-ACV and SMS-SB pre-BCR null by using CRISPR/CAS9 gene editing with guide RNAs specific for the hypervariable region (recombined V, D, and J segments) of their expressed IgH allele. As identified by flow cytometry for the pre-BCR, deficient RCH-ACV and SMS-SB cells exhibited reduced viability and impaired proliferation when compared to their pre-BCR+ controls (Figure 1). Pre-BCR- cells showed reduced baseline phosphorylation of CD19, VAV1 and AKT. Interestingly, BTK and ERK phosphorylation were not affected. These results provide evidence for the dependency of pre-BCR+ ALL on pre-BCR signaling and suggest selective involvement of the PI3K-AKT pathway.
We also investigated the effects of pharmacological pre-BCR inhibition by treating pre-BCR+ and pre-BCR- ALL cell lines and xenograft expanded primary patient samples with PRT318, a small-molecule inhibitor of spleen tyrosine kinase (SYK). In pre-BCR+ ALL PRT318 blocked cell proliferation and selectively inhibited AKT phosphorylation, thus mimicking the effects of IgH knockout. Pre-BCR- ALL cells were resistant to PRT318.
Key effectors of the pre-BCR during normal B-cell development are FOXO transcription factors. In line with this, we found reduced FOXO1 phosphorylation and increased FOXO1 total protein levels after IgH knockout as well as after treatment with PRT318. This was accompanied by an increase in the FOXO1 transcriptional targets p27 and BLNK, suggesting increased FOXO1 transcriptional activity in response to the inhibition of pre-BCR signaling. To study the contribution of FOXO1 to the effects of IgH knockout and SYK inhibition more thoroughly, we expressed constitutively active FOXO1 (FOXO1-3A) in the pre-BCR+ ALL cell line RCH-ACV and consequently assessed its effects on cell proliferation and protein expression. Similar to IgH knockout and PRT318, FOXO1-3A reduced cell proliferation and increased p27 and BLNK protein levels, confirming FOXO1 as an important downstream target of pre-BCR signaling in B-ALL.
To identify additional effectors of the pre-BCR in B-ALL we performed gene expression profiling (GEP) to compare pre-BCR+ and pre-BCR- cells of RCH-ACV and SMS-SB. Gene set enrichment analysis (GSEA) showed that IgH knockout resulted in significant enrichment for gene sets associated with down-modulation of MYC activity. This was confirmed by Western blot analysis of MYC total protein levels, and consistent with the finding of reduced MYC protein in PRT318-treated and FOXO1-3A-expressing pre-BCR+ cells, all indicating that pre-BCR signaling modulates MYC activity through a mechanism involving SYK and FOXO1.
In conclusion, we provide evidence for the dependence of certain B-ALL subgroups on pre-BCR signaling. According to our data this is mainly due to pre-BCR-induced inactivation of FOXO1 and the subsequent deregulation of MYC. Importantly, pharmacological inhibition of pre-BCR signaling with the SYK inhibitor PRT318 completely reversed these effects, therefore providing a rationale for the use of SYK inhibitors in pre-BCR+ subgroups of B-ALL.
Coffey:Portola Pharmaceuticals: Employment, Equity Ownership.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal