Abstract
Background: Blastic plasmacytoid dendritic cell neoplasm (BPDCN) is a rare malignancy of plasmacytoid dendritic cells as classified by the World Health Organization (WHO), and is characterized by distinct clinical, pathological and genetic features. BPDCN used to be diagnosed as blastic NK-cell lymphoma or CD4+/CD56+ hematodermic neoplasm. Its prognosis is extremely poor with a median overall survival (OS) of about 1 year. Allogeneic hematopoietic stem cell transplantation (allo-HSCT) in remission has shown encouraging results for patients with BPDCN. However, the role of high-dose chemotherapy followed by autologous stem cell transplantation (HDT/ASCT) has not been reported.
Methods: We surveyed the data of the Transplant Registry Unified Management Program of the Japan Society for Hematopoietic Cell Transplantation and identified 109 patients with an original diagnosis of BPDCN, blastic NK-cell or lymphoblastic lymphoma of NK-cell lineage. These diagnoses had been made by a pathologist at each institution in accordance with the WHO classification or previous lymphoma classification systems. Then, we sent out questionnaires to collect additional data on clinical presentation, prior therapies and update of follow-up, in addition to pathology and flow cytometry reports. The diagnosis of BPDCN was made with clinicopathological review by a consensus panel.
Results: Of the 109 identified patients, additional data were obtained for 83. After central clinicopathological review, 58 patients were excluded from analysis due to disease other than BPDCN or the absence of important clinical data. Finally, the diagnosis of BPDCN was confirmed in 25 patients (allo-HSCT, N = 14; HDT/ASCT, N = 11).
The median age at HSCT was 58 (range, 17-67) years and male patients were predominant (80%). Involvements of skin and bone marrow at diagnosis were observed in 88% and 68% of patients, respectively. The induction chemotherapy regimen was as follows: non-Hodgkin lymphoma (NHL)-like (e.g. cyclophosphamide, doxorubicin, vincristine, prednisone [CHOP]-like regimens; or ifosfamide/etoposide-based regimens) (N = 11), acute lymphoblastic leukemia (ALL)-like (N = 10) and acute myeloid leukemia (AML)-like (N = 4). The rate of initial response to induction chemotherapy was high, with a complete remission (CR) rate of 96%, except for one patient who experienced progressive disease in the central nervous system during induction therapy with an ALL-like regimen.
The median time from diagnosis to HSCT was 6 months (range, 2-22). Among the patients who underwent allo-HSCT, myeloablative (MAC) and reduced-intensity (RIC) regimens were used in 8 (57%) and 6 (43%) patients, respectively. The donor of 1st allogeneic transplant was a relative in 7, unrelated bone marrow in 7 and cord blood in 1. Regarding the disease status at transplantation, all 11 patients underwent HDT/ASCT in CR1 whereas, among patients who underwent allo-HSCT, 12 were in CR1 (N=10)/CR2 (N =2) and two had disease.
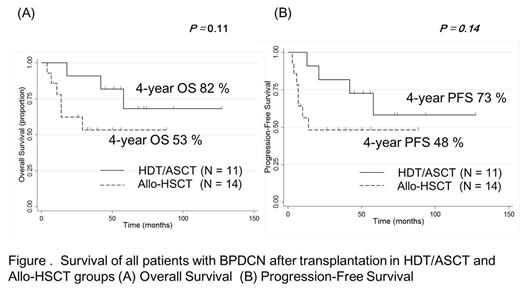
With a median follow-up of 53.5 months in surviving patients, OS and progression-free survival (PFS) at 4 years were 65% and 58%, respectively (Figure 1). The OS at 4 years for patients who received HDT/ASCT and allo-HSCT were 82% and 53%, respectively (P = 0.11, Figure 2). The PFS at 4 years for patients who received HDT/ASCT and allo-HSCT were 73% and 48%, respectively (P = 0.14, Figure 2). The three induction regimen groups (NHL-like, ALL-like and AML-like regimens) had similar OS rates: 62%, 70% and 67% at 4 years, respectively (P = 0.86). OS did not differ significantly between MAC and RIC groups (OS at 4 years: 45% vs. 60%, P = 0.31).
There was no non-relapse mortality within 100 days after HSCT. Seven patients (28%) relapsed at a median of 10 months (range, 3-21) after HSCT (MAC allo-HSCT, N = 2; reduced-intensity conditioning allo-HSCT, N = 3; HDT/ASCT, N = 2). Notably, there was no relapse in patients without bone marrow (BM) infiltration at diagnosis in the HDT/ASCT treatment group. After allo-HSCT, grade 2-4 acute GVHD was observed in 5 patients (20%).
Conclusions: As previous reports have shown, HSCT can achieve long-term survival in patients with BPDCN. In addition, the result of HDT/ASCT in CR1 seems to be promising and deserves further evaluation in the settings of prospective trials. Meanwhile, we need to clarify who could be cured by ASCT and need to improve outcome for high-risk patients, such as those with BM invasion.
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal