Abstract
Reconstitution of T cell function after allogeneic HSCT is dependent on the balanced recovery of CD4+Foxp3+ regulatory T cells (Treg) and CD4+Foxp3- conventional T cells (Tcon). While Tcon are required for effector T cell function, Treg play an essential role in the maintenance of immune tolerance after allogeneic HSCT and prevention of graft versus host disease (GVHD). To examine the reconstitution of Treg and Tcon after HSCT and identify mechanisms that contribute to homeostatic imbalance of these T cell subsets, we undertook a prospective analysis of 188 adult patients (median age 54y) with hematologic malignances who underwent T-cell replete allogeneic HSCT. Patients received either myeloablative (MAC; n=80) or reduced-intensity conditioning (RIC; n=108). GVHD prophylaxis differed in these two cohorts as RIC patients received tacrolimus/methotrexate/sirolimus-based regimens and MAC patients received tacrolimus/methotrexate-based regimens. Serial blood samples (total n=739) obtained at 1, 2, 3, 6, 9 and 12 months after transplant were characterized by flow cytometry with a panel of intracellular and surface markers designed to quantify phenotypically and functionally distinct subsets of Treg, Tcon and CD8 T cells in each sample.
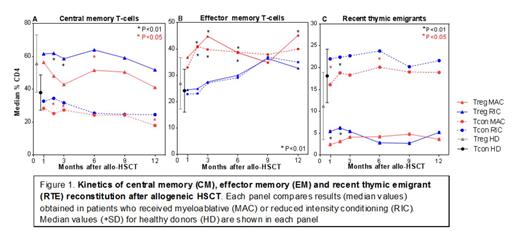
Likely reflecting the prophylactic administration of sirolimus for the first 6 months post-HSCT after RIC conditioning, recovery of absolute CD3+, CD4+ and CD8+ T cell counts was significantly greater after MAC-HSCT throughout the first year after HSCT. Total CD4+ Tcon recovery was significantly decreased in RIC patients at all time points but Treg recovery was significantly lower only in the first 2 months after HSCT. Central memory cells (CM; 45RA-62L+) comprise the majority of Treg and Tcon throughout the first year after HSCT. The percentage of Treg-CM is greater than Tcon-CM and the fraction of CM cells within Treg and Tcon is increased in the RIC cohort (Figure 1A). Effector memory cells (EM; 45RA-62L-) also represent a large fraction of Treg and Tcon. Reconstitution of Treg-EM and Tcon-EM is similar but recovery of this subset appears to be strongly affected by sirolimus (Figure 1B). In RIC patients who receive sirolimus, the percentage of EM cells within Treg and Tcon subsets is significantly lower in the first 6 months after HSCT. Naïve Treg and Tcon (CD45RA+CD62L+) represent a relatively small fraction of recovering CD4 T cells during this period. Reconstitution of naïve CD4 T cells identified as recent thymic emigrants (RTE; CD45RA+CD31+) is markedly different in Treg and Tcon (Figure 1C). Within Tcon, the RTE fraction rapidly recovers to levels observed in healthy donors. In contrast, the fraction of RTE-Treg remains very low, with no evidence of improvement for at least 1 year. For both Treg-RTE and Tcon-RTE, recovery is significantly greater after RIC suggesting that either the reduced-intensity of conditioning or sirolimus is thymus protective.
In vivo proliferation of each T cell subset was monitored by expression of Ki67. As previously observed in healthy donors, proliferation of Treg was significantly greater than Tcon at all time points. This primarily reflects homeostatic proliferation of Treg memory cells since very few naïve Treg are present. Both Treg and Tcon proliferation was higher in the MAC cohort in the first 1-3 months after HSCT. In vivo susceptibility to apoptosis was monitored by expression of pro-survival Bcl-2 and pro-apoptotic CD95 (FAS) expression. All Treg subsets expressed lower levels of Bcl-2 and higher levels of CD95 compared to Tcon. RIC/sirolimus was associated with higher levels of Bcl-2 and lower levels of CD95 predominately in the first 3 months after transplant. This effect was evident in all Treg and Tcon subsets.
These results demonstrate distinctly different patterns of reconstitution of Treg and Tcon after allogeneic HSCT. Reconstitution of Tcon is characterized by rapid recovery of thymic generation, moderate homeostatic proliferation of memory subsets and relative resistance to apoptosis. Reconstitution of Treg is characterized by prolonged impaired thymic generation, high levels of homeostatic proliferation of memory subsets and increased susceptibility to both intrinsic and extrinsic pathways of apoptosis. RIC followed by administration of sirolimus for GVHD prophylaxis appears to selectively delay recovery of Treg and Tcon EM cells while sparing naïve, RTE and CM subsets.
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal