Abstract
Introduction: Double-unit cord blood (CB) transplantation (dCBT) has been investigated to augment engraftment in patients without an adequately dosed single CB unit. However, only one unit engrafts in the majority of patients and the role, if any, of the non-dominant unit is not established.
Methods: To investigate whether the non-engrafting unit facilitates engraftment of the dominant unit, we studied 129 patients (median age 34 years, 76% > 16 years) who underwent myeloablative dCBT for hematological malignancies at our center in 10/2005-8/2013. Chimerism testing at 21 and 28 days after dCBT was used to identify dominant and non-dominant CB units which could be assigned even in cases of clinical graft failure based on bone marrow chimerism.
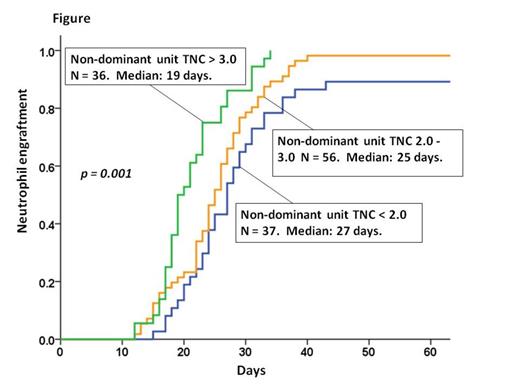
Results: The cumulative incidence of neutrophil engraftment at day 45 was 95% (95%CI: 90-98) and occurred at a median of 24 days (range 12-43). The dominant CB unit was the only unit detected in the peripheral blood by day 28 in 111 patients (86%) whereas both the dominant and non-dominant units were detected in the remaining 18 patients [median non-dominant unit contribution to chimerism 20% (range 6-48)]. Recipients of units that had close unit-unit HLA- allele match (7-9/10 vs 1-6/10) were more likely to have both CB units detected at day 28 (27% vs 9%, respectively, p = 0.018). However, there was no engraftment advantage associated with persistence of the non-dominant unit 21-28 days post-dCBT with a median neutrophil recovery of 27 days (range 14-34) in those co-engrafting with both units vs 24 days (range 12-43) in those engrafting with a single unit. In univariate analyses, dominant unit infused total nucleated cell (TNC) dose, infused viable CD34+ cell dose, infused viable CD3+ cell dose, infused viable CD3-56+16+ cell dose, and percentage of viable CD34+ cells post-thaw (CD34+ cell viability) were all significantly associated with neutrophil engraftment. However, the TNC (Figure) and CD3+ cell doses of the non-dominant unit were also associated with neutrophil engraftment on univariate analysis [hazard ratio (HR) 1.35, p < 0.001 and HR 1.16, p < 0.001, respectively] whereas non-dominant unit CD34+ cell and CD3-56+16+ cell doses were not (HR 1.18, p = 0.107 and HR 1.06, p = 0.159, respectively). Neither donor-recipient HLA-match of dominant or non-dominant units, nor unit-unit HLA-match, were associated with time to neutrophil engraftment. Notably, on multivariate analysis, the dominant unit infused viable CD34+ cell dose, dominant unit CD34+ cell viability, and the non-dominant unit infused TNC dose were each independently associated with the speed and success of neutrophil engraftment (Table). To further assess potential effects of the non-dominant unit TNC dose, we evaluated the subset of patients in whom haematopoiesis by day 28 was purely derived from the dominant unit. Among these 111 patients, non-dominant unit TNC dose remained independently associated with engraftment in multivariate analysis (HR 1.21, p = 0.026). Finally, we hypothesized that the non-dominant unit TNC dose effect may be seen primarily in patients whose dominant units had a relatively low infused viable CD34+ dose. Indeed, analysis revealed the non-dominant unit TNC effect was only evident in the two-thirds of patients (n = 86) whose dominant unit infused viable CD34+ cell dose was low (< 1.20 x 105/kg, HR 1.37, p = 0.006). There was no association in patients whose dominant unit had a higher infused viable CD34+ cell dose (HR 1.03, p = 0.799).
Conclusions: While infused viable CD34+ cell dose of the dominant unit is critical, the non-dominant unit TNC dose was also independently associated with neutrophil engraftment after dCBT. Therefore, despite their lack of engraftment, non-dominant units may facilitate engraftment of the dominant units in myeloablative dCBT recipients. These data suggest dCBT is associated with interactions that foster engraftment of the dominant unit. This finding supports continued investigation of dCBT, especially in adults who are more likely to receive units with low CD34+ cell doses. Furthermore, the mechanisms that underlie this observation warrant investigation.
| Table (n = 129 dCBT recipients) | Multivariate Analysis | ||
| HR | 95% CI | p value | |
| Dominant unit viable CD34+ cell x 105/kg (continuous) | 1.72 | 1.41-2.10 | < 0.001 |
| Dominant unit CD34+ cell viability (%) | 1.03 | 1.00-1.05 | 0.026 |
| Non-dominant unit TNC x 107/kg (continuous) | 1.19 | 1.01-1.40 | 0.035 |
| Table (n = 129 dCBT recipients) | Multivariate Analysis | ||
| HR | 95% CI | p value | |
| Dominant unit viable CD34+ cell x 105/kg (continuous) | 1.72 | 1.41-2.10 | < 0.001 |
| Dominant unit CD34+ cell viability (%) | 1.03 | 1.00-1.05 | 0.026 |
| Non-dominant unit TNC x 107/kg (continuous) | 1.19 | 1.01-1.40 | 0.035 |
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.
This icon denotes a clinically relevant abstract

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal