Abstract
Introduction: Leukemic stem cells (LSCs) are recognized as important mediators of leukemia relapse. Thus, therapeutic antibodies are in development to target antigens present on these cells. The success of this approach relies on a detailed understanding of the surface marker expression patterns of LSCs. To address this, we applied multi-parametric single-cell mass cytometry (MCM) to deeply profile the surface marker expression of all major immunophenotypic populations in bone marrow aspirates (BM) from patients with acute myeloid leukemia (AML).
Methods: BMs from 41 leukemia patients (30 AML, 4 APL, 2 high-risk MDS, 5 AML in CR) and 5 healthy donors and were processed immediately after aspiration (<1min) and stored for pooled analysis with two overlapping 39-antibody MCM panels (50 markers total). All samples were barcoded, such that 20 samples (leukemia and healthy) could be combined into a single tube for simultaneous antibody staining and analysis, resulting in high precision (coefficient of variation = 10-20%).
Results: Distinct AML subtype-specific patterns of cell frequencies across immunophenotypic populations were detected. Patients with core binding factor (CBF) AML (n=5) and those with adverse-risk karyotpic abnormalities (n=6) exhibited the greatest expansion of immunophenotypic hematopoietic stem cells (HSCs) and early progenitors (MPP, CMP), while patients with APL (n=4) and FLT3-ITD normal karyotype AML (NK-AML; n=11) exhibited reduced expansion of early progenitors and expansion in more mature myeloid progenitors (GMP, myelo-monoblasts).
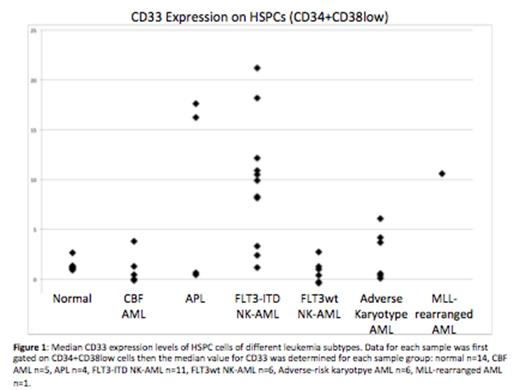
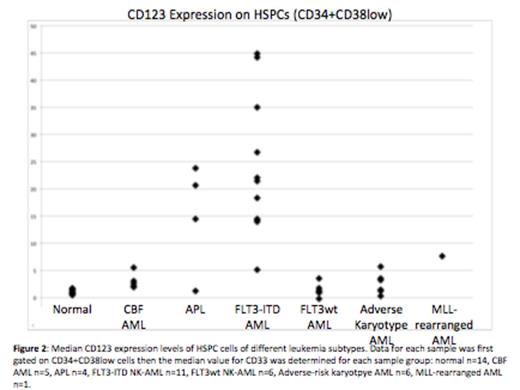
As a result of barcoding, high resolution (2-3 fold changes) measurement of surface marker expression detected multiple aberrancies across almost all identifiable immunophenotypic populations. Specifically, several genotype- and karyotype-specific trends in aberrant marker expression were observed in hematopoietic stem and progenitor cell populations (HSPCs; CD34+CD38low). FLT3-ITD samples were characterized by increased CD7, CD33, CD123, CD45, CD321, and CD99, as well as decreased CD34, CD117, and CD38. FLT3wt NK-AML samples (n=6) were characterized by increased CD99 as well as decreased CD71, CD47, CD34, and CD45. Adverse-risk karyotype samples (n=6) were characterized by increased CD99 and decreased CD47. All p values were significant (ranging from 0.02 to 4.5x10-7).
While all 36 samples (AML or APL) displayed immunophenotypic abnormalities within the CD34+CD38low gated population (3.6 abnormalities on average), there were 9 samples in which an unambiguous separation into normal and leukemic populations was observed among the cells in this gate. Interestingly, among these samples, some markers were aberrantly high in leukemic cells of one subtype, and aberrantly low in leukemic cells of another, e.g. HLA-DR was extremely high in HSPCs of sample #22 (MLL rearrangement), and aberrantly low in all 4 APL samples. CD99 was most consistently elevated in the AML samples (28/36), but was normal in 6 samples, including a majority of those with t(8;21).
These results suggest that therapeutic antibodies directed against molecules such as CD33 may be less effective for AML subtypes such as FLT3wt NK-AML (~40% decrease in CD33), and more effective for other subtypes such as FLT3-ITD NK-AML (7-fold increase in CD33). HSPCs from patients with FLT3-ITD mutations also displayed a 20-fold increase in CD123, which could also be therapeutically targeted. These findings, combined with our recent observation that HSPCs from FLT3-ITD NK-AML patients have an extremely low S-phase fraction, may provide a mechanistic basis for the improved disease-free survival recently reported for FLT3-ITD NK-AML patients treated with fractionated gemtuzumab ozogamicin in combination with standard therapy.
Conclusions: We have developed an innovative MCM approach for the analysis of hematologic malignancies and demonstrated that different genotypic and karyotypic subsets of AML are highly varied in their immunophenotypic properties, particularly within the stem and progenitor cell compartment. These data also suggest that antigens that distinguish AML stem cells from normal stems cells are likely to be karyotype and genotype-specific. These findings have important implications for the design of therapeutic strategies in AML.
Behbehani:Fluidigm: Consultancy. Medeiros:Agios: Consulting - Ad board Other. Nolan:Fluidigm, Inc: Consultancy, Equity Ownership.
Author notes
Asterisk with author names denotes non-ASH members.


This feature is available to Subscribers Only
Sign In or Create an Account Close Modal