Abstract
Acute lymphoblastic leukemia (ALL) has a high relapse rate in adults. While the biology of the patient may be responsible for the marked difference in survival seen in comparison to children, variable adherence to complex chemotherapy regimens may also play a role. However, there is very little understanding about the risk factors for delays in therapy and the impact of delays on survival.
To study delays in newly diagnosed adult ALL patients, we conducted an observational study using data from ECOG 2993/ MRC_UKALLXII (Rowe, Blood 2005). We analyzed Ph- patients who started intensification after documented complete remission (CR). A long delay (LD) was defined as > 98 days from start of induction to start of intensification (IS), which was >2 weeks delay beyond the 84 days recommended per protocol. A Very Long Delay (VLD) was defined as a >4 weeks delay.
Of 2109 patients enrolled, 1247 patients met inclusion criteria for analysis. Of note, 435 Ph- patients who achieved CR after induction but did not proceed to intensification were excluded. In univariate analysis, female sex (p<0.001), Black race (p=0.01), and older age (p<0.001) were associated with increased odds of LD. During induction presence of infection (p=0.01), dose reductions (p=0.001), duration of neutropenia (p=0.007), thrombocytopenia (p<0.001), and hospitalization duration (p<0.001) were associated with LD. In multivariate regression, age, female sex, dose reduction, Black race, and hospitalization duration were significantly associated with LD and VLD (Table I).
At 2 years after diagnosis, 801/1247 (64.2%) patients were alive. Of the surviving patients, 181 (22.5%) had VLD, and 620 (77.4%), had delay <4 weeks, p=0.073. Of the alloHCT patients, 333 were alive: 57/333 (17%) had VLD, and 276/333 (83%) had been delayed <4 weeks (p=0.036).
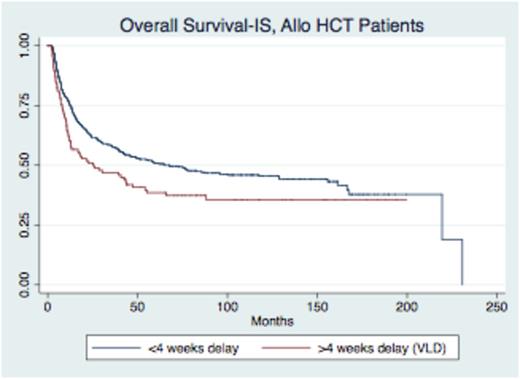
As of July 2014, 687/1247 (55%) patients have died, with a median time to death from start of intensification of 13.3 months (range 0.8-231). Survival analysis was stratified by post-remission therapy, with living patients censored at date last seen. Patients who received myeloablative allogeneic HCT (allo HCT) had poorer overall survival from start of intensification (OS-IS) and event free survival (EFS-IS) after LD or VLD. While OS-IS and EFS-IS were significantly worse for alloHCT patients after VLD (p=0.02 and p=0.03) respectively, survival was not worse for non-transplant patients after VLD (p=0.24 and p=0.10) (Figure I).
In a multivariate Cox regression, adjusting for other high-risk disease features (age, cell lineage, and white cell count at diagnosis), patients who underwent allo HCT had significantly worse OS-IS (HR 1.4, p=0.02) and EFS-IS (HR 1.4, p=0.02) after experiencing VLD compared to alloHCT patients who experienced <4 weeks delay. When evaluating OS and EFS from diagnosis, VLD was still associated with poorer OS (HR 1.34, p=0.04) and EFS (HR 1.34, p=0.03). There was no difference in OS or EFS in patients who received non-transplant post-remission therapy based on delay.
In a comprehensive analysis of the largest adult ALL study ever reported, we identified significant risk factors predictive of LD and VLD (>2 and >4 weeks beyond mandatory rest period). Our findings highlighted that patients were delayed because of chemotherapy toxicity and also identified healthcare disparities. VLD was associated with poorer OS and EFS in patients undergoing alloHCT, but not in patients undergoing non-transplant post remission therapy. Further studies are planned to prospectively identify patient barriers to on-schedule treatment with a goal of earlier intervention. The ability to better predict and intervene on risk factors for delay can improve adherence to protocol and optimize long-term survival.
Multivariate Regression for LD and VLD Risk Factors
| Variable . | Odds Ratio . | 95% Confidence Interval . | p-value . |
|---|---|---|---|
| Days in the hospital Phase I Induction | 1.01 | 1.00-1.02 | 0.04 |
| Duration of Thrombocytopenia, Phase I | 1.02 | 1.01-1.03 | 0.001 |
| Age by Decade | 1.2 | 1.08-1.31 | <0.001 |
| Reduced Dose in Induction | 1.54 | 1.08-2.22 | 0.019 |
| Sex, Female | 1.56 | 1.22-2.0 | <0.001 |
| Race, Black | 3.4 | 1.41-7.96 | 0.006 |
| Variable . | Odds Ratio . | 95% Confidence Interval . | p-value . |
|---|---|---|---|
| Days in the hospital Phase I Induction | 1.01 | 1.00-1.02 | 0.04 |
| Duration of Thrombocytopenia, Phase I | 1.02 | 1.01-1.03 | 0.001 |
| Age by Decade | 1.2 | 1.08-1.31 | <0.001 |
| Reduced Dose in Induction | 1.54 | 1.08-2.22 | 0.019 |
| Sex, Female | 1.56 | 1.22-2.0 | <0.001 |
| Race, Black | 3.4 | 1.41-7.96 | 0.006 |
p=0.023
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal