Abstract
Background
Several models predict the progression from smouldering multiple myeloma (SMM) to therapy requiring multiple myeloma (MM). Three models comprise the assessment of tumour mass by different clinical parameters to stratify in risk groups: 1) the Mayo Clinic model uses bone marrow plasma cells percentage (BMPC) and serum monoclonal protein (M-protein), 2) the PETHEMA model uses immunoparesis and the percentage of abnormal plasma cells by flow cytometry, 3) the Heidelberg group assesses tumour mass by either the percentage of malignant plasma using iFISH or the Mayo assessment depicted above, and the presence of chromosomal aberrations associated with adverse prognosis. Besides tumor mass, they find the number of focal lesions in whole body MRI (>1) as strong prognostic factor.
Aim
To assess a combination of easily accessible clinical factors identifying patients at ≥ 80% risk of progression to MM requiring treatment within two years from the diagnosis of SMM.
Methods
Data for this study were obtained from the Registry of Monoclonal Gammopathies (RMG) acquired from hematologic centers of the Czech Republic for 287 SMM patients enrolled from May 2007 to June 2013. A cohort comprising 240 SMM patients from Heidelberg, Germany was used for validation (Neben et al. JCO 2013).
Results
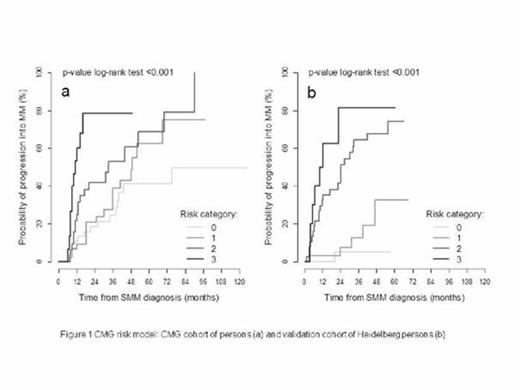
During the follow up period (median 2.4 years; range 0.6 - 18.0) progression to MM was observed in 51.9% (149/287) patients in the study cohort, representing 16% risk of progression at 1 year, 31.2% at 2 years, 54.8% at 5 years and 73.4% at 10 years. In univariate analysis factors significantly associated with progression were as follows: serum free light chain (iFLC/uFLC) ratio > 30 (HR 2.4 [95% CI: 1.4 - 4.1]; p< 0.001) plasma cell infiltration in bone marrow cytology ≥ 15% (HR 2.1 [1.5-3.0]; p< 0.001), immunoparesis (HR 2.0 [1.3-2.9]; p< 0.001), M - protein concentration ≥ 2.3 g/dL (HR 2.00 [1.4-2.7]; p< 0.001), beta2 microglobulin ≥ 2.0 mg/l (HR 1.8 [1.2-2.7]; p= 0.001), and thrombocyte count ≤ 250 x 109/l (HR 1.7 [1.1-2.4]; p= 0.005). In multivariate analysis, 3 parameters showed independent predictive value (immunoparesis, serum M-protein quantity ≥ 2.3 g/dL and iFLC/uFLC > 30). Combining these factors, we proposed a new risk model for SMM patients (CMG model). The risk of progression from SMM to MM at 2 years was 18.5%, 20.9%, 41.9% and 78.7% if 0 (reference group), 1, 2 or 3 risk factors are present (p< 0.001) (Figure 1) with HR of 1.5 [0.7-2.9]; p=0.283, 2.5 [1.3-5.0]; p= 0.008, 6.8 [3.0-15.2]; p<0.001, n=139), respectively. The CMG model was validated on 240 SMM patients from Heidelberg published in 2013. The risk of progression from SMM to MM at 2 years was 5.3%, 7.5%, 44.8% and 81.3% if 0, 1, 2 or 3 risk factors were present, respectively (p< 0.001) (Figure 1) with HR of 4.2 ([0.5-36.1]; p=0.189), 21.5 ([2.9-159.1]; p= 0.003, HR 38.6 [4.7- 317.7]; p<0.001, n=113).
Conclusion
We propose and validate a new risk model for SMM patients with prediction of 80% (78.7% on our CMG model; 81.3% on data from Heidelberg) risk of progression to therapy requiring myeloma within two years based on easily accessible clinical parameters (CMG model). The model could especially be used to identify high-risk patients to be included in early treatment clinical trials.
Acknowledgments: This work was supported by grants NT13492-4, NT14575-3 and by EU FP7/2007-2013; grant n°278570 and “OverMyR”, as well as the Deutsche Forschungs-Gemeinschaft (DFG) SFB/TRR79.
CMG risk model: CMG cohort of patients and validation cohort of Heidelberg patients
CMG risk model: CMG cohort of patients and validation cohort of Heidelberg patients
Seckinger:Novartis: Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal