Abstract
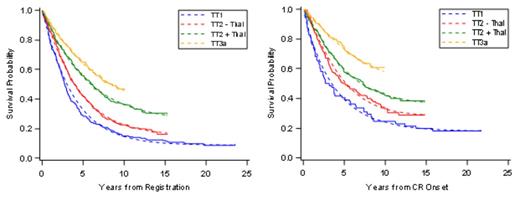
Intra-tumoral heterogeneity is a hindrance to curing malignant disease. By employing all MM-active drugs upfront, TT was designed to overcome such obstacle by targeting all potential MM sub-clones broadly. We are reporting on long-term results of phase-2 TT1, phase-3 TT2 and phase-2 TT3a trials, with median follow-up times of 21yr, 12yr and 9yr, respectively. Five year estimates of OS, PFS and CR duration (CRD) increased from 58%, 28% and 40% with TT1 to 65%, 42% and 50% with TT2’s control arm (TT2-), to 68%, 56% and 58% with TT2’s thalidomide arm (TT2+), and to 74%, 65% and 74% with TT3a (all p<0.0001). The 5-yr estimates of time to progression (TTP)/time to relapse (TTR) from CR decreased from 59%/58% in TT1 to 43%/42% in TT2-, to 28%/34% in TT2+, and to 22%/18% in TT3a (all p<0.0001). When examined in the era of gene expression profiling (GEP) of purified plasma cells, 5-yr estimates of TTP/TTR in GEP70 low-risk MM were 47%/46% with TT2-, 26%%/36% with TT2+, and 18%/16% with TT3a (all p<0.0001); the corresponding data for the 15% with high-risk MM were 60%/50% with TT2-, 62%/64% in TT2+, and 48%/35% in TT3a (NS). Relative survival (RS) was computed per year in relationship to age- and sex-matched controls. RS ratios approached 1 at 10–15 years for TT1 and TT2-, but earlier, at 5-10 years, for TT2+ and TT3a. A parametric mixture cure model was used to estimate PFS and CR duration for each protocol, from baseline and from a 5-year landmark. The cure model fits the data well and provides cure-fraction estimates that increase with later protocols (Figure 1). Importantly, when comparing data from GEP-defined low-risk and high-risk MM, plateaus for both PFS- and CRD-based cure fractions emerged at 10 years in the former and earlier, at 5 years, in the latter (data not shown). PFS-based cure-fraction estimates increasedsignificantly with successive TT trials: 9% in TT1, 16% in TT2-, 25% in TT2+, and 33% in TT3a (p=0.04). CRD-based cure-fraction estimates were 18%, 28%, 36%, and 49%, respectively (p=0.17) (Table 1). When a 5-year landmark was applied to exclude early myeloma-related events, PFS-based cure fraction estimates were 28% in TT1, 39% in TT2-, 51% in TT2+, and 70% in TT3a (p<0.001); in this setting, CRD-based cure fraction estimates were 32%, 47%, 56%, and 75%, respectively (p=0.007). MRD flow cytometry was available in 83 of 175 TT2 PFS patients, of whom 78% were MRD-. Our TT results are consistent with curability of MM. Solely novel agent-based trials without transplant lack the long-term follow-up to determine their cure potential.
As MM usurps the bone marrow micro-environment (ME) for its growth and survival, we reasoned that ME alterations reflecting an angiogenic switch may persist beyond the state of clinical CR and even MRD. We therefore performed GEP analyses of bone marrow biopsies in CR and compared findings with age- and sex-matched controls. Indeed, biopsy “normalization” was linked to superior CR duration and is being developed as an early “cure surrogate” marker to help define the length of maintenance therapy in future trials. Although about 50% of CR patients treated with TT3a can be considered cured, the median PFS in high-risk MM of 2yr has not improved with successive TT trials. Reasoning that cytokine storms emanating after myelosuppressive therapies to restore normal hematopoiesis may be growth-stimulatory to MM cells, we applied non-myelotoxic metronomic therapy (Papanikolaou, Haematologica, 2014). Six newly diagnosed transplant-ineligible high-risk patients were offered an extended 28-day course of low-dose metronomic therapy with bortezomib, thalidomide, dexamethasone and continuous infusions of doxorubicin and cisplatin, to which were added arsenic trioxide and vincristine infusions. Remarkably, 5 of 6 achieved CR status after a single course and remain disease-free without further therapy 6-8 months later. Our results attest to the curability of MM with TT and, given the short PFS of 2yr in high-risk MM, such patients should be the focus of future novel interventions that yield information in a timely manner.
Cure fraction estimates
| Protocol . | PFS . | CR duration . | ||
|---|---|---|---|---|
| N . | Cure Fraction . | N . | Cure Fraction . | |
| From start of therapy | ||||
| TT1 | 231 | 8.8% | 79 | 17.9% |
| TT2 -Thal | 345 | 15.5% | 146 | 28.2% |
| TT2 +Thal | 323 | 25.1% | 200 | 35.6% |
| TT3a | 303 | 32.9% | 189 | 48.8% |
| From 5-yr landmark | ||||
| TT1 | 65 | 28.4% | 33 | 32.3% |
| TT2 -Thal | 145 | 39.2% | 84 | 47.4% |
| TT2 +Thal | 150 | 51.1% | 134 | 55.9% |
| TT3a | 197 | 69.8% | 148 | 74.7% |
| Protocol . | PFS . | CR duration . | ||
|---|---|---|---|---|
| N . | Cure Fraction . | N . | Cure Fraction . | |
| From start of therapy | ||||
| TT1 | 231 | 8.8% | 79 | 17.9% |
| TT2 -Thal | 345 | 15.5% | 146 | 28.2% |
| TT2 +Thal | 323 | 25.1% | 200 | 35.6% |
| TT3a | 303 | 32.9% | 189 | 48.8% |
| From 5-yr landmark | ||||
| TT1 | 65 | 28.4% | 33 | 32.3% |
| TT2 -Thal | 145 | 39.2% | 84 | 47.4% |
| TT2 +Thal | 150 | 51.1% | 134 | 55.9% |
| TT3a | 197 | 69.8% | 148 | 74.7% |
van Rhee:Janssen: Membership on an entity's Board of Directors or advisory committees; Celgene: Membership on an entity's Board of Directors or advisory committees; Millenium: Membership on an entity's Board of Directors or advisory committees; Sanofi: Membership on an entity's Board of Directors or advisory committees. Heuck:Celgene: Honoraria; Foundation Medicine: Honoraria; Millennium: Membership on an entity's Board of Directors or advisory committees; Janssen: Membership on an entity's Board of Directors or advisory committees.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal