Abstract
Introduction:
Maintenance lenalidomide after autologous hematopoietic stem cell transplantation (auto HCT) has been shown to improve progression free (PFS) and overall survival (OS) in myeloma patients. In this analysis we sought to determine the impact of lenalidomide treatment on achievement of complete remission (CR), survival and the incidence of secondary primary malignancies (SPM).
Methods:
We retrospectively analyzed all (N=466) consecutive myeloma patients who underwent auto HCT and received maintenance lenalidomide between August 2006 and September 2013 at our institution. Patients received doses of maintenance lenalidomide ranging from 5 mg – 15 mg/day or every other day. We looked at whether lenalidomide improved disease status, specifically CR in patients who had not achieved this before maintenance initiation and the median time to achieve CR. We also analyzed the effects of early initiation (<4-months after auto HCT) of maintenance lenalidomide versus late initiation (≥ 4-months after auto HCT) with regards to PFS and OS using the Kaplan-Meier method. Lastly we assessed if continuation of maintenance therapy beyond 2 and 3-years after auto HCT improved PFS and OS and increased the incidence of SPM.
Results:
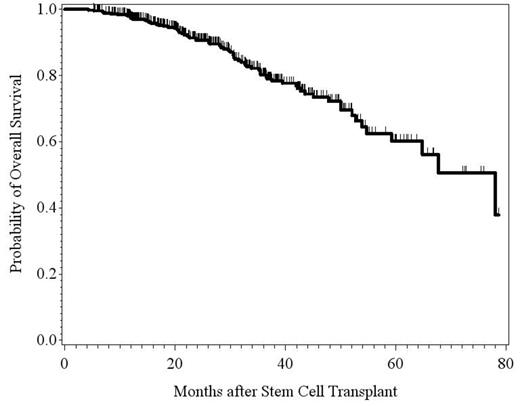
The median follow-up time for all patients was 26.6 months. 173 patients (37%) experienced improvements in their disease status. Of these, 86 patients (50% of those with noted improvements and 19% of total patients assessed) who were not in CR before maintenance achieved CR while on maintenance. The average time to achieve CR in these patients was 12.9 months. Comparing the patients who were started on early maintenance treatment with those who were started on late maintenance therapy, we did not find any difference with regards to PFS (Hazard Ratio [HR]=0.90; p-value=0.57) and OS (HR=0.90; p-value=0.74). However, patients who had been on lenalidomide for > 2 years experienced a significant benefit in OS compared with those on lenalidomide for ≤ 2 years (HR=0.36; p-value=0.0015), although no difference in PFS was observed between the two cohorts (HR=0.77; p-value=0.18). A similar trend in OS was seen for patients who had been on lenalidomide > 3 years compared with those on maintenance treatment ≤ 3 years, though not statistically significant (HR=0.47; p-value=0.10). Again, no difference in PFS was noted between the two groups. Lastly there were only 12 cases of SPM reported in all patients assessed with no statistically significant association to the duration of lenalidomide use. In all 12 cases, lenalidomide was suspended and the mortality among these patients was 50%.
Conclusions:
Maintenance lenalidomide improves response rates after auto HCT in some patients including the CR rate, however, the median time to achieve CR in these patients is approximately 13 months. The patients who received maintenance therapy for > 2years had a significantly lower risk of death with a trend of improved OS in patients who continued maintenance therapy beyond 3-years. We conclude that the maintenance therapy should be continued for at least 2 years and possibly longer after auto HCT.
Summary of Survival Outcomes
| . | Maintenance Treatment Initiation . | . | |
|---|---|---|---|
| Measure . | Early (N=155) . | Late (N=184) . | p-value . |
| Progression-free survival | |||
| Hazard ratio (95% CI)a | 0.90 (0.63, 1.30) | 0.57 | |
| Overall survival | |||
| Hazard ratio (95% CI)a | 0.90 (0.49, 1.66) | 0.74 | |
| Duration of Maintenance Treatment | |||
| Measure | > 2 years (N=115) | ≤ 2 years (N=224) | p-value |
| Progression-free survival | |||
| Hazard ratio (95% CI)b | 0.77 (0.52, 1.13) | 0.18 | |
| Overall survival | |||
| Hazard ratio (95% CI)b | 0.36 (0.19, 0.67) | 0.0015 | |
| > 3 years (N=49) | ≤ 3 years (N=290) | p-value | |
| Progression-free survival | |||
| Hazard ratio (95% CI)b | 0.75 (0.45, 1.26) | 0.28 | |
| Overall survival | |||
| Hazard ratio (95% CI)b | 0.47 (0.19, 1.15) | 0.10 | |
| . | Maintenance Treatment Initiation . | . | |
|---|---|---|---|
| Measure . | Early (N=155) . | Late (N=184) . | p-value . |
| Progression-free survival | |||
| Hazard ratio (95% CI)a | 0.90 (0.63, 1.30) | 0.57 | |
| Overall survival | |||
| Hazard ratio (95% CI)a | 0.90 (0.49, 1.66) | 0.74 | |
| Duration of Maintenance Treatment | |||
| Measure | > 2 years (N=115) | ≤ 2 years (N=224) | p-value |
| Progression-free survival | |||
| Hazard ratio (95% CI)b | 0.77 (0.52, 1.13) | 0.18 | |
| Overall survival | |||
| Hazard ratio (95% CI)b | 0.36 (0.19, 0.67) | 0.0015 | |
| > 3 years (N=49) | ≤ 3 years (N=290) | p-value | |
| Progression-free survival | |||
| Hazard ratio (95% CI)b | 0.75 (0.45, 1.26) | 0.28 | |
| Overall survival | |||
| Hazard ratio (95% CI)b | 0.47 (0.19, 1.15) | 0.10 | |
a Cox proportional hazards regression model: measure included as a baseline covariate with the following additional covariates: patient’s cytogenetic risk, creatinine, hemoglobin, B2-microglobulin, ISS stage at diagnosis, disease status at auto HCT, and response prior to auto HCT.
b Cox proportional hazards regression model: measure included as a time-dependent covariate with the covariates listed above.
Comparison of Overall Survival in Early vs Late initiation of Revlimid
Shah:Novartis: Consultancy, Research Funding; Millennium Pharmaceuticals: Consultancy, Research Funding; Celgene: Consultancy, Research Funding; Onyx Pharmaceuticals: Consultancy, Research Funding; Array: Consultancy, Research Funding. Orlowski:Onyx Pharmaceuticals: Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; Millennium Pharmaceuticals: Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.


This feature is available to Subscribers Only
Sign In or Create an Account Close Modal