Abstract
BACKGROUND: BH3 profiling is a functional assay that assesses the degree to which cells are primed to die via mitochondrial apoptosis. We previously used this technique to show that pre-treatment CLL cells from patients who responded to frontline chemoimmunotherapy had higher levels of apoptotic priming than cells from refractory patients (Davids et al., Blood, 2012). BH3 profiling also detects dependence on individual anti-apoptotic Bcl-2 family proteins. Recently, ABT-199, a selective, potent, small molecule antagonist of the anti-apoptotic protein Bcl-2, has been found to be highly active in patients with relapsed or refractory CLL, even those with high-risk markers such as del(17p) (Seymour et al., EHA, 2014). Unlike its Bcl-XL/Bcl-2-targeting predecessor navitoclax (ABT-263), ABT-199 spares platelets, which rely on Bcl-XL for survival. We hypothesized that baseline levels of apoptotic priming would be associated with the depth of initial clinical response to ABT-199 in CLL patients.
METHODS: Peripheral blood samples were obtained just prior to initial dosing on the phase I, first-in-human study of ABT-199 in relapsed/refractory CLL. Viability was determined by Annexin V–FITC and propidium iodine by FACS. BH3 profiling was performed by exposing gently permeabilized CLL cells to a panel of BH3-domain peptides, fixing the cells, and quantifying apoptotic priming by assessing cytochrome C release by FACS. Platelets from 3 healthy donors were also analyzed. Clinical response was assessed by comparing baseline values to first-re-staging, as follows: peripheral blood: % reduction in absolute lymphocyte count, lymph node: % reduction in the sum of the product of the diameters of the 6 target lesions, and bone marrow: % reduction in morphologic bone marrow intertrabecular space involvement. Linear regression was used to assess clinical responses as continuous variables, and non-parametric Mann-Whitney testing was used for intergroup comparisons. Two-tailed p values were used in all cases.
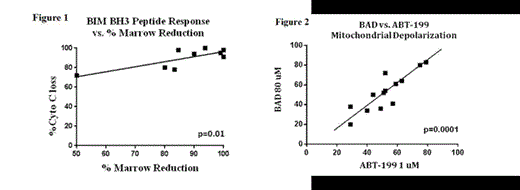
RESULTS: Ex vivo treatment of platelets with navitoclax induced substantial apoptotic priming, whereas minimal baseline priming was unchanged after ex vivo treatment with ABT-199. Baseline blood samples from 14 relapsed/refractory CLL patients on the ABT-199 phase I study were analyzed for viability after ex vivo treatment with ABT-199 and for pre-treatment levels of apoptotic priming. With ex vivo treatment, ABT-199 induced dose-dependent apoptosis, with a median EC50 of 23 nM in CLL cells (n=14); however, the ABT-199 EC50 was not associated with clinical response in blood, lymph node, or bone marrow. In contrast, samples with higher levels of apoptotic priming as measured by BIM BH3 peptide had a significantly deeper response in the bone marrow (Figure 1, n=9, p=0.01) and blood (n=13, p = 0.05), with a trend toward better response in lymph node (n= 14, p = 0.19). Reflecting the observation in the clinic that ABT-199 has an equivalent response rate irrespective of del(17p) status, we found no difference in apoptotic priming in patients with (n=5) or without (n=9) del(17p). Interestingly, although patients who were fludarabine-refractory at study entry (n=8) were highly primed at baseline, they had lower levels of apoptotic priming (median 92% depolarization) than those who were not refractory (n=6) (median 98%) (p=0.04). To provide further validation of the on target effect of ABT-199 on Bcl-2 on CLL mitochondria, we also studied low concentration ABT-199 used like a BH3 peptide in the BH3 profiling assay. We found an excellent correlation between the amount of mitochondrial depolarization caused by ABT-199 and BAD BH3 peptide (Figure 2, n=13, p = 0.0001).
CONCLUSIONS: BH3 profiling provides valuable insights into the mechanism of action of the promising Bcl-2 antagonist ABT-199. In contrast to traditional EC50 assays, the pre-treatment level of apoptotic priming predicts initial clinical response to ABT-199 in CLL. An analysis of the association between apoptotic priming and progression free survival is ongoing. With additional validation, BH3 profiling may eventually guide optimal patient selection for Bcl-2 directed therapy, and potentially for other therapies in CLL.
Davids:Genentech: Consultancy; Infinity Pharmaceuticals: Consultancy. Brown:Sanofi, Onyx, Vertex, Novartis, Boehringer, GSK, Roche/Genentech, Emergent, Morphosys, Celgene, Janssen, Pharmacyclics, Gilead: Consultancy. Letai:Abbvie: Consultancy, Research Funding; Tetralogic: Consultancy, Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal