Abstract
Introduction: The importance of miRNAs in regulating gene expression has been addressed in several haematological cancers including myelodysplastic syndrome (MDS). In MDS, miR-145 and miR-146a have been associated with some of phenotypic features of 5q- syndrome. Monosomy 7/7q deletion is the second most common chromosomal abnormality in MDS and identifies a subgroup of patients with poor prognosis. Thus far, no studies have examined the role of miRNA dysregulation in MDS patients with monosomy 7. In this study we examined the functional consequences of deletion of miRNAs localized to 7q with particular focus on miR-595, which is localized to 7q36.3, which is commonly deleted region in 7q- and -7.
Most of the available methodologies for miRNA target identification are based on computational algorithms. Therefore, there is a relatively high incidence of false positive target identification. To enable the identification of biologically relevant microRNA targets, a novel functional assay was developed in our lab, which is based on positive/negative selection (Gaken et al, 2012). Using this assay, we identified functional target of miR-595 and correlated this with the MDS phenotype.
Method: The functional assay relies on miR-595, identified several targets including RPL27A, HSPA14, GRM5 and SEC63. We focused on ascertaining the biological function of RPL27A. RPL27A was validated as a target for miR-595, by examining the changes on RPL27A expression using quantitative RT-PCR and western blot analysis in cells transfected with plasmid expressing miR-595. We demonstrated a targeted reduction of RPL27A in several cell lines including HCT-116 and HEL cells (expressing p53) as well as HCT-116 p53-/- and K562 cells (not expressing p53) through retroviral expression of (shRNAs). RPS14 knocked down was used as a control. The knockdown of RPL27A and RPS14 was also performed in normal CD34+ (n=6) in two stages liquid culture using cytokines mixture to induce erythroid, myeloid and megakaryocyte differentiation. The effects on expressing erythroid cells were assessed and composed of CD71 and GlyA, myeloid and megakaryocyte cells using CD11b and CD41, respectively.
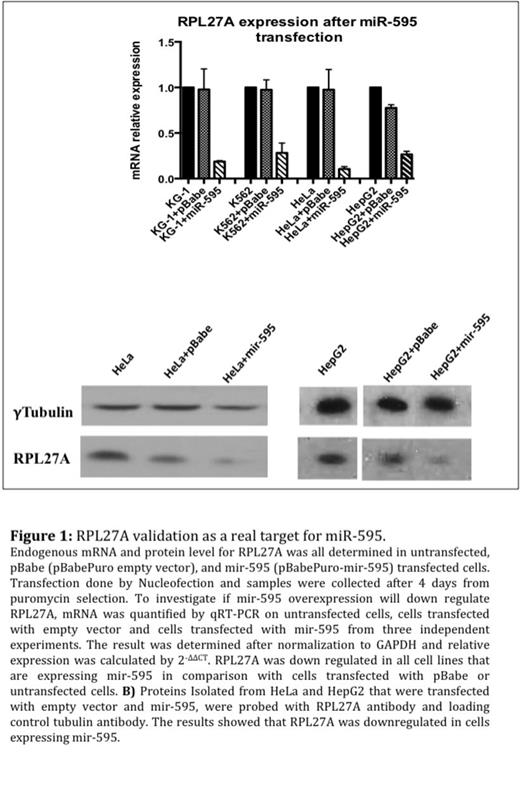
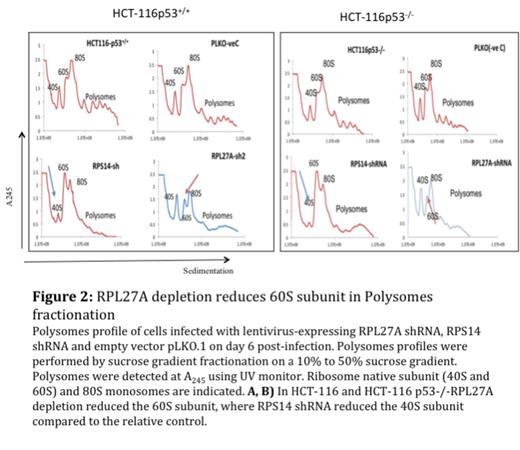
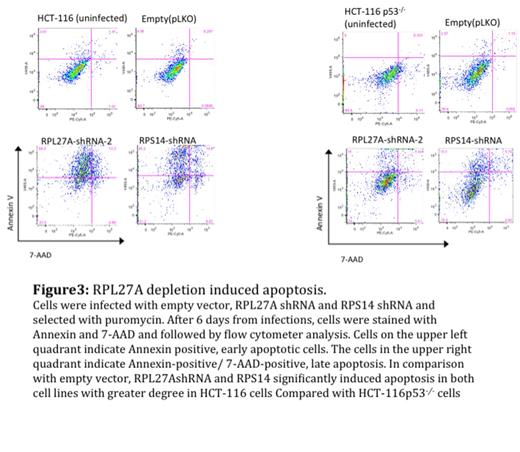
Result: RPL27A was validated as target for miR-595 (Figure1). RPL27A knockdown resulted in a reduction in the large ribosomal subunit (60S) based on Polysomes fractionation, (Figure 2). On day 6 of the knockdown, the depletion of RPL27A led to p53 activation and induced apoptosis in all cell lines at different levels, 40-60% increases in apoptotic cells in HCT-116 and HEL cell lines and 20-30% increases in apoptotic cells in HCT-116 p53-/- and K562 cells (Figure 3). Interestingly, like RPS14 knockdown, the effect of RPL27A knockdown in 48 h was p53 dependent. The p53 independent effect, which was observed on day 6, was attributed to a defect in ribosome biogenesis based on the disruption of nucleolar staining in HCT-116 and HCT-116 p53-/- with depleted RPL27A using fibrillarin antibody and similar effects were seen with RPS14.
To investigate whether the effect of RPL27A knockdown on erythroid differentiation is similar to the other ribosomal proteins, RPL27A knockdown (approximately 70% reduction at day 6) was performed in normal CD34+ and resulted in reduce viability and apoptosis within 10 days. Post-transcriptional p53 overexpression was also observed; this was associated with a marked increase in the mRNA expression of p53 targets, p21 and Bax. Following 10 days in liquid culture, RPL27A knockdown blocked the proliferation and differentiation of erythroid cells relative to myeloid and megakaryocyte lineages. Similar observations have been made in 5q- syndrome due to RPS14 haploinsufficiency and in DBA patients with mutations in large or small ribosomal subunit proteins.
Conclusion: This study showed that haploinsufficency of miR-595 in patients with -7/7q del is important in pathogenesis of reduced erythropoiesis and a reduction in myeloid and erythroid progenitor populations. The effects are mediated via RPL27A, which is a ribosomal protein and its knockdown produces it effects via p53 dependent pathways, leads to ineffective erythropoiesis and arrest of the myeloid and megakaryocyte differentiation. These effects are particularly relevant in patients with complex chromosomal abnormalities that include haploinsufficiency of RPS14 due to -5/del 5q.
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal