Abstract
Background: Hematopoietic stem cells (HSCs) constitute a rare population of bone marrow cells (BMCs) and their ex vivo expansion with stemness for a prolonged period is quite unlikely. It is also difficult to efficiently produce HSCs from embryonic stem cells (ESCs) and induced pluripotent stem cells (iPSCs). HoxB4 is a positive regulator of murine HSC self-renewal when ectopically expressed. Moreover, it was reported that HoxB4 overexpression could promote differentiation of ESCs to definitive HSCs. We established iPSCs (GGH-iPSCs) from BMCs of a C57BL/6J-Ly5.2 GATA2-GFP knock-in mouse (PNAS 103:2202, 2006) and transduced them with a 4-hydroxytamoxyfen (4-HT)-inducible HoxB4 construct. Using GGH-iPSCs, we showed that enforced HoxB4 activity throughout their culture period resulted in the long-term maintenance of iPSC-derived HSCs, which could repopulate in recipient mice over 30 weeks after transplantation, in vitro (Blood 122:2418, 2013). In the present study, we tried to elucidate the precise stage of hematopoietic differentiation governed by enforced HoxB4 in this iPSC model, especially in association with GATA2 expression.
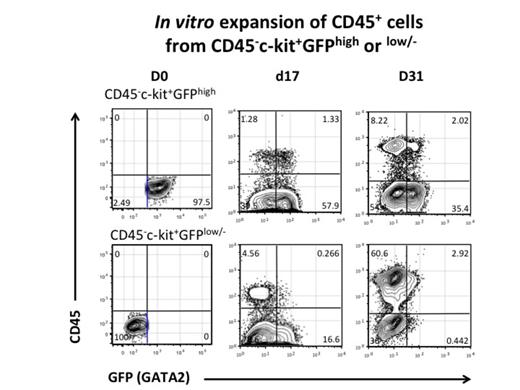
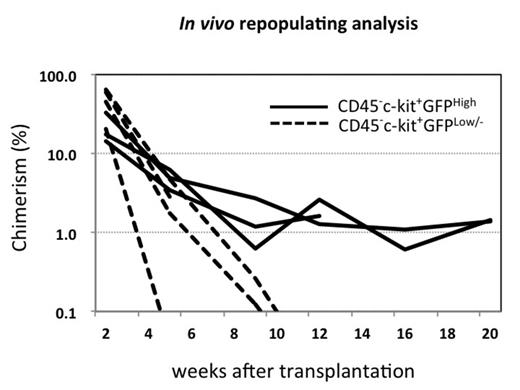
Experimental procedures and Results: GGH-iPSCs were induced toward hematopoietic differentiatin under enforced HoxB4, and after 2 months of culture, almost all the resulting cells expressed c-kit but not CD45, and approximately half of them were highly positive for GFP (GATA2). We also found that these CD45- cell populations expressed HSC marker genes such as SCL and LMO2, and that they produced CD45+ blood cells after HoxB4 was switched off. These results suggest that CD45- pre-HSCs, which can develop LT-HSCs, may be included in HoxB4-sustained iPSC-derived cell populations. Next, according to the GFP (GATA2) expression level, we divided CD45-c-kit+ cells on HoxB4 into two fractions; CD45-c-kit+GATA2high and CD45-c-kit+GATA2low/- cells, and cultured FACS-sorted cells over an OP9 monolayer with cytokines and without 4-HT to switch off HoxB4. Then, CD45+ blood cells emerged from both cell fractions over time, and CD45-c-kit+GATA2low/- cells produced much more CD45+ cells than CD45-c-kit+GATA2high cells one month later (Figure Left). Furthermore, to test a long-term repopulating activity in mice, each of cell fractions was cultured without 4-HT for 4 days after sorting, and then subjected to transplanttion into sublethally-irradiated C57BL/6-Ly5.1 recipient mice. Two weeks after transplantation, peripheral blood Ly5.2+ donor cells were much more frequent in mice transplanted with CD45-c-kit+GATA2low/- cells, compared to those with CD45-c-kit+GATA2high cells, whereas the CD45-c-kit+GATA2low/- fraction-derived donor cells rapidly decreased and disappeared around 2 months later. In contrast, CD45-c-kit+GATA2high donor cells could repopulate in recipient mice over 20 weeks after transplantation (Figure Right). These results suggest that CD45-c-kit+GATA2low/- and CD45-c-kit+GATA2high cell fractions include short-term (ST) and long-term (LT) -repopulating HSCs, respectively.
Conclusion: Taken together, in a hematopoietic differentiation model of murine GGH-iPSCs, long-term repopulating hematopoietic stem cells (LT-HSCs) emerge from CD45-c-kit+GATA2high pre-HSC fraction which can be sustained in vitro by enforced HoxB4. In this experimental setting, enforced HoxB4 is likely to hinder differentiation from CD45- pre-HSCs to CD45+ HSCs.
Tojo:Novartis: Research Funding, Speakers Bureau; Bristol-Myers Squibb: Research Funding; Chugai: Research Funding, Speakers Bureau; Dainippon Sumitomo: Research Funding; Pfizer: Research Funding; Celgene: Consultancy.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal