Abstract
Introduction: In normal conditions, circulating red blood cells (RBCs) are not supposed to adhere. Some erythroid adhesion molecules expressed on young reticulocytes are therefore lost upon maturation. Conversely, abnormal RBC adhesiveness in sickle cell anemia (SCA) through activation, sustained or increased expression of adhesion molecules are considered central in vaso occlusive crisis (VOC), the hallmark of SCA.
SCA is seldom symptomatic in the first six months of life. One main explanation lies in the sustained level of fetal hemoglobin (HbF) and F cells during this period preventing HbS polymerization. However, infra clinical vaso occlusion, particularly in the spleen, has been demonstrated to be present and the absolute reticulocyte count is paradoxically elevated in the first semester of life, evidencing the very early onset of hemolysis.
Our objectives were to analyze the profile of erythroid adhesion molecules in SCA and non-SCA infants in combination with HbF level, in order to evidence significant differences specifically attributable to SCA, informative on very early pathophysiology and disease severity.
Patients and methods: Infants were enrolled in a longitudinal multi center prospective study on prognostic factors in SCA between September 2010 and March 2013. Blood sampling was performed at enrolment (3-6 months) at steady state, in asymptomatic infants. In parallel, blood samples from infants with no hemoglobinopathy were collected. Flow cytometer analysis (BD FACSCanto II) was performed using murine monoclonal antibodies against CD36, CD44, CD47, CD49d, CD58, CD99, CD147, CD239 and CD242. Statistical analysis was performed with Graphpad software, Prism 6. Differences in the percentage of positive cells and the level of expression of molecules between groups were calculated with Mann Whitney test. Results were considered statistically significant at an a-risk level of 5%.
Results:
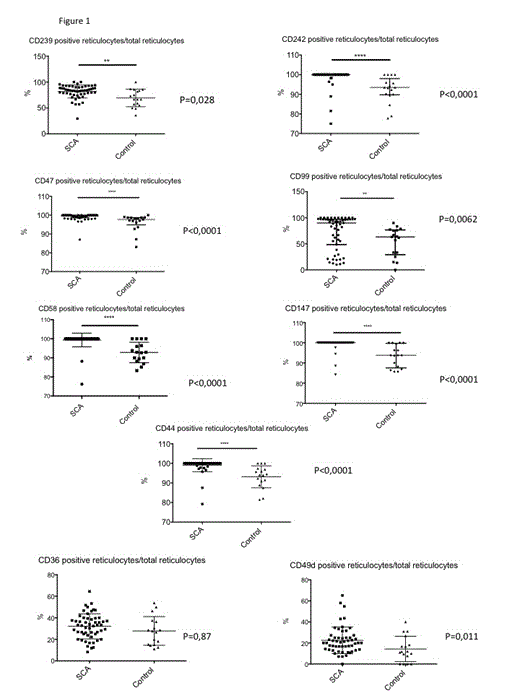
Fifty-four SCA infants were included and compared to 17 non-SCA infants. Median age in the two groups was not statistically different (144 days, range 81-196 versus 128, range 68-621, p=0,84). Mean HbF level was, as previously described, statistically increased in SCA compared to non-SCA infants (41.2% +/-11.2 versus 10,4 % +/- 1,8; p<0,0001). Median reticulocyte count tended to be more elevated in SCA infants (2.6 % range 0,5-10) compared to non-SCA infants (2 range 1-3,1) without reaching statistical significance (p=0,052).
Reticulocytes from SCA infants display a distinct surface molecule expression profile, with a statistically increased percentage of reticulocytes expressing the following adhesion molecules: CD239 (Lu/BCAM), CD242 (ICAM-4), CD47, CD99, CD58, CD147 and CD44 (Figure 1). Furthermore the mean intensity of fluorescence is statistically increased concerning CD239, CD58 and CD242 (data not shown). Surprisingly, known adhesion markers demonstrated to play an important role in SCA pathogeny i.e. CD36 and CD49d (α4β1/Very Late Antigen-4) are not significantly increased in SCA infants (Figure 1)
No significant difference of expression profile was found on mature or total RBCs between SCA and non-SCA infants (data not shown).
Discussion: Recent publications have pointed to the significance of reticulocytosis associated with an increased risk of death or stroke during childhood. Our results further demonstrate that this early rise of reticulocyte count in SCA infants, despite elevated HbF level, include reticulocytes with a specific adhesion molecule expression profile. This numerically small subpopulation of red cells may play in fact a major role in this complex disease. An interesting hypothesis may be that the spleen, as long as its function is preserved, prevents VOC by trapping this subpopulation in very young infants. As spleen function decreases, proadhesive reticulocytes remain in the circulation, which may, in combination with the decline of HbF, subsequently favor extra splenic VOC.
Ongoing analysis will confirm if this idiosyncratic erythroid adhesion molecule profile in infants indeed correlates with HbF level and, importantly, correlates with functional activation, in order to serve as a readily available biomarker of disease severity.
De Montalembert:Novartis: Speakers Bureau. Le Van Kim:SHIRE: Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal