Abstract
Background: AML is characterized by molecular heterogeneity; morphology and genetic alterations are important prognostic factors (Weinberg, 2009). In the large, phase 3, multicenter, randomized AZA-AML-001 study, AZA treatment (Tx) prolonged median overall survival (OS) vs CCR by ~4 months (10.4 vs 6.5 mo; p=0.1009) in older pts with newly diagnosed AML (>30% BM blasts). About 33% of patients (pts) in AZA-AML-001 had AML with morphologic dysplastic changes (AML-MDC).
Aim: Determine effects of AZA vs CCR on OS, response, and safety in the subset of pts with AML-MDC in the AZA-AML-001 trial; and further analyze OS in AML-MDC pts who had been preselected to receive low-dose cytarabine (LDAC) before randomization to AZA or CCR.
Methods: Eligible pts were age ≥65 years (yrs), had AML with >30% BM blasts, ECOG performance status (PS) 0-2, WBC count ≤15x109/L, and intermediate- or poor-risk cytogenetics. This analysis includes pts who, based on local assessment, had AML-MDC.Before randomization, investigators preselected the preferred Tx option for each pt from 3 CCR: intensive chemotherapy (IC; standard 7 + 3 regimen), LDAC (20mg SC BID x 10 days [d]/28d cycle), or best supportive care (BSC) only. Pts were then randomized to AZA (75mg/m2/d x 7 d/28d cycle) or CCR, and then received the preselected CCR. Median OS (Kaplan-Meier method) and percent of pts alive at 1 yr were compared between AZA vs CCR for all pts with AML-MDC, and between AZA vs LDAC specifically in pts with AML-MDC preselected to LDAC before randomization. Response was assessed by IWG AML criteria (Cheson, 2003). Transfusion independence (TI) was defined as no transfusion for 56 consecutive days for transfusion dependent (TD) pts at baseline (ie, ≥1 transfusion in 56d before randomization). Overall response was defined as complete remission (CR) + morphologic CR with incomplete blood count recovery (CRi). Adverse events (AEs) were graded by NCI-CTCAE v4. Hazard ratios (HR) and 95% confidence intervals (95%CI) were determined by unstratified Cox proportional hazards model and p values from log-rank or Fisher's exact test.
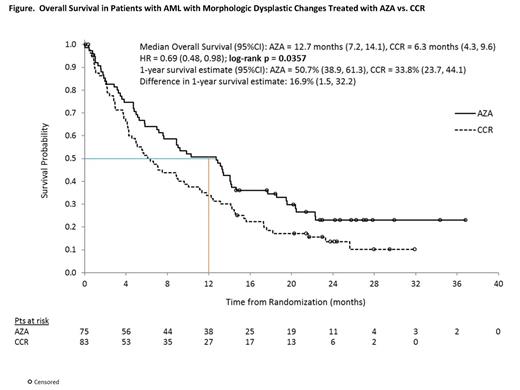
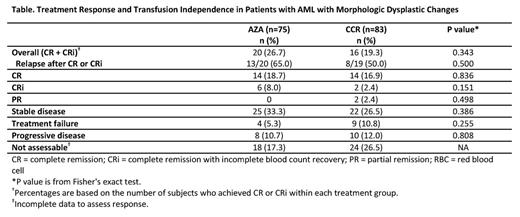
Results: Of 488 pts in AZA-AML-001, 158 (32.4%) had AML-MDC and are included in these analyses. Baseline characteristics were generally balanced between Tx arms (AZA n=75, CCR n=83 [IC n=13, LDAC n=50, and BSC n=20]). Median (range) ages were 76 (65, 86) and 74 (66, 87) yrs in the AZA and CCR groups, respectively. Higher proportions of pts in the AZA arm were age ≥75 yrs (59% vs 48% of CCR) and had prior MDS (47% vs 37%). In the AZA and CCR arms, 43% and 47% of pts had poor-risk cytogenetics, and 24% and 25% had ECOG PS score of 2. Median (range) %BM blasts (central review) in the AZA and CCR arms was 67% (4, 99) and 73% (9, 100). Mediannumber of Tx cycles with AZA, IC, and LDAC were 6 (1, 28), 1 (1, 3), and 3 (1, 23). Median exposure to BSC was 79 d (8, 535). Median OS in pts with AML-MDC was prolonged 2-fold with AZA vs CCR: 12.7 mo (95%CI 7.2, 14.1) vs 6.3 mo (4.3, 9.6); HR=0.69 (0.48, 0.98), p=0.0357 (Figure). Similarly, 1-yr survival was greater with AZA: 50.7% vs 33.8% with CCR (16.9% difference [95%CI 1.5, 32.2]). Rates of CR+CRi were 26.7% with AZA vs 19.3% with CCR (Table). Most pts with AML-MDC (n=99, 63%) were preselected to receive LDAC (AZA n=49, LDAC n=50). Median OS in the preselected LDAC group with AZA vs LDAC, respectively, was 13.2 mo vs 6.3 mo, a 6.8-mo improvement with AZA (HR=0.76 [95%CI 0.49, 1.19], p=0.23). Further, 1-yr survival with AZA vs LDAC was 55% vs 31% (24% difference [95%CI 5.0, 43.0]). In the overall AML-MDC group with baseline-TD, 16/51 (31%) and 14/58 (24%) attained RBC TI in the AZA and CCR groups, and 10/26 (39%) and 12/33 (36%) attained platelet TI. Grade 3-4 anemia, neutropenia, febrile neutropenia, and thrombocytopenia rates, respectively, were: AZA 12%, 30%, 30%, 28%; IC 15%, 46%, 39%, 31%; LDAC 16%, 29%, 35%, 27%; and BSC 6%, 11%, 44%, 11%.
Conclusions: AZA prolonged median OS 2-fold to >1 yr in this older, poor-risk subgroup of pts with AML-MDC. More than one-half of AZA-treated pts with AML-MDC remained alive at 1 yr. Notably, median OS in pts preselected to LDAC, but who were randomized to AZA, was more than doubled vs pts who did receive LDAC. AZA safety profile was consistent with that seen in the entire AZA-AML-001 population (Dombret, 2014). Compared with commonly used CCR, AZA was safe, effective and well- tolerated in this subset of pts with AML. These data suggest AZA may be a beneficial initial Tx as an alternative to LDAC in pts with AML-MDC.
Seymour:Celgene: Consultancy, Honoraria, Speakers Bureau. Off Label Use: Use of azacitidine in AML with blast count >30%. Döhner:Celgene: Consultancy. Wierzbowska:Celgene: Honoraria, Speakers Bureau. Selleslag:Celgene: Consultancy, Research Funding, Speakers Bureau. Cavenagh:Celgene: Honoraria. Kumar:Celgene: Honoraria, Membership on an entity's Board of Directors or advisory committees. Schuh:Celgene: Membership on an entity's Board of Directors or advisory committees. Candoni:Celgene: Consultancy, Speakers Bureau. Récher:Celgene: Membership on an entity's Board of Directors or advisory committees, Research Funding. Sandhu:Celgene: Honoraria. Bernal del Castillo:Celgene: Consultancy. Al-Ali:Celgene: Honoraria, Research Funding. Martinelli:Novartis: Consultancy, Speakers Bureau; BMS: Consultancy, Speakers Bureau; Pfizer: Consultancy; ARIAD: Consultancy. Falantes:Celgene: Consultancy. Stone:Agios: Consultancy; AbbVie: Consultancy; Amgen: Consultancy; Celator: Consultancy; Celgene: Consultancy; Roche: Consultancy. Minden:Celgene: Honoraria. McIntyre:Celgene: Employment. Songer:Celgene: Employment, Equity Ownership. Lucy:Celgene: Employment, Equity Ownership. Beach:Celgene: Employment, Equity Ownership. Dombret:Celgene: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees.
Author notes
Asterisk with author names denotes non-ASH members.
This icon denotes a clinically relevant abstract

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal